Eye drops containing olopatadine hydrochloride and preparation method of eye drops
A technology of olopatadine hydrochloride and eye drops, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, which can solve the problems of dry eyes, sodium hyaluronate, etc. Unstable, easy to be polluted and long-term bacteria, etc., to reduce the occurrence of complications and sequelae, relieve dry eyes, and relieve eye fatigue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
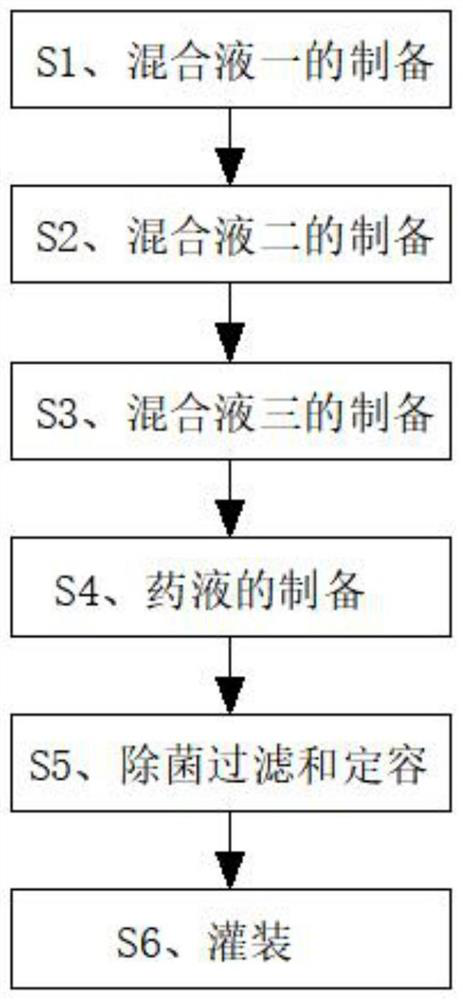
[0027] Please refer to figure 1 As shown, the invention discloses an eye drop containing olopatadine hydrochloride, comprising the following raw materials in parts by weight: 0.3 parts of olopatadine hydrochloride, 0.3 parts of hypromellose, 0.4 parts of bacteriostatic agent, 0.1 part of thickener, 0.4 part of pH regulator, 0.7 part of osmotic pressure regulator, 0.6 part of water-soluble antioxidant and 80 parts of water for injection.
[0028] A preparation method of eye drops containing olopatadine hydrochloride, comprising the following steps:
[0029] S1. Preparation of mixed solution 1: Weigh the required olopatadine hydrochloride in parts by weight, put it into a container, and add an appropriate amount of water for injection to dissolve to obtain mixed solution 1, which is set aside;
[0030] S2. Preparation of mixed solution 2: Weigh the required hypromellose, bacteriostat and thickener in parts by weight and put them into the liquid mixing tank. The thickener is pol...
Embodiment 2
[0036] Please refer to figure 1 As shown, the invention discloses an eye drop containing olopatadine hydrochloride, comprising the following raw materials in parts by weight: 0.45 parts of olopatadine hydrochloride, 2.2 parts of hypromellose, 0.5 parts of bacteriostatic agent, 0.2 part of thickener, 0.5 part of pH regulator, 0.8 part of osmotic pressure regulator, 0.67 part of water-soluble antioxidant and 90 parts of water for injection.
[0037] A preparation method of eye drops containing olopatadine hydrochloride, comprising the following steps:
[0038] S1. Preparation of mixed solution 1: Weigh the required olopatadine hydrochloride in parts by weight, put it into a container, and add an appropriate amount of water for injection to dissolve to obtain mixed solution 1, which is set aside;
[0039] S2. Preparation of mixed solution 2: Weigh the required hypromellose, bacteriostatic agent and thickener in parts by weight and put them into the liquid mixing tank. The thicke...
Embodiment 3
[0045] Please refer to figure 1 As shown, the invention discloses an eye drop containing olopatadine hydrochloride, comprising the following raw materials in parts by weight: 0.6 part of olopatadine hydrochloride, 0.5 part of hypromellose, 0.7 part of bacteriostatic agent, 0.3 part of thickener, 0.6 part of pH regulator, 0.9 part of osmotic pressure regulator, 0.7 part of water-soluble antioxidant and 96 parts of water for injection.
[0046] A preparation method of eye drops containing olopatadine hydrochloride, comprising the following steps:
[0047] S1. Preparation of mixed solution 1: Weigh the required olopatadine hydrochloride in parts by weight, put it into a container, and add an appropriate amount of water for injection to dissolve to obtain mixed solution 1, which is set aside;
[0048] S2. Preparation of mixed solution 2: Weigh the required hypromellose, bacteriostat and thickener in parts by weight and put them into the liquid mixing tank. The thickener is chitos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com