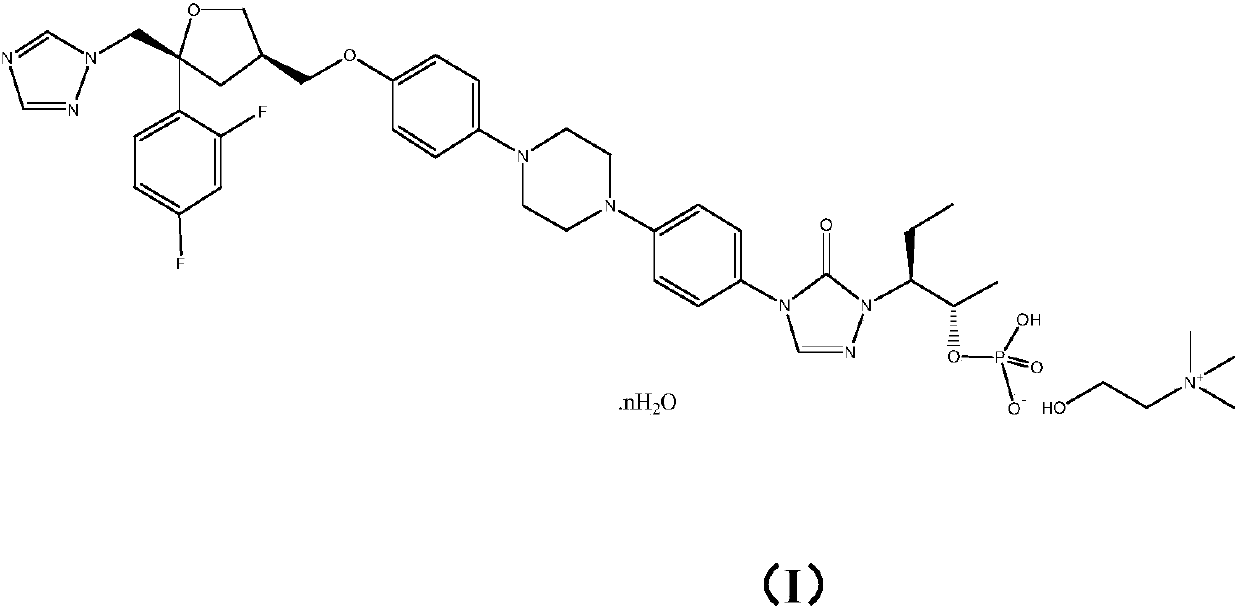
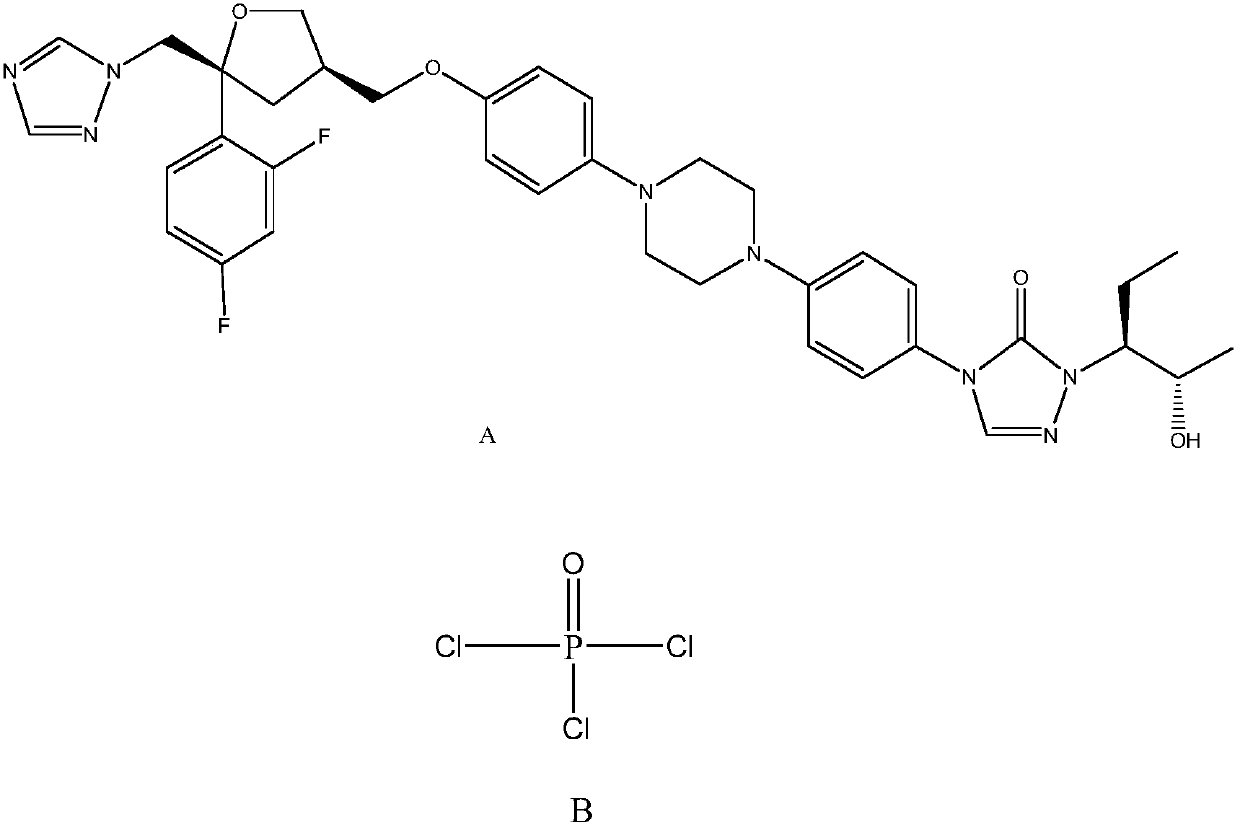
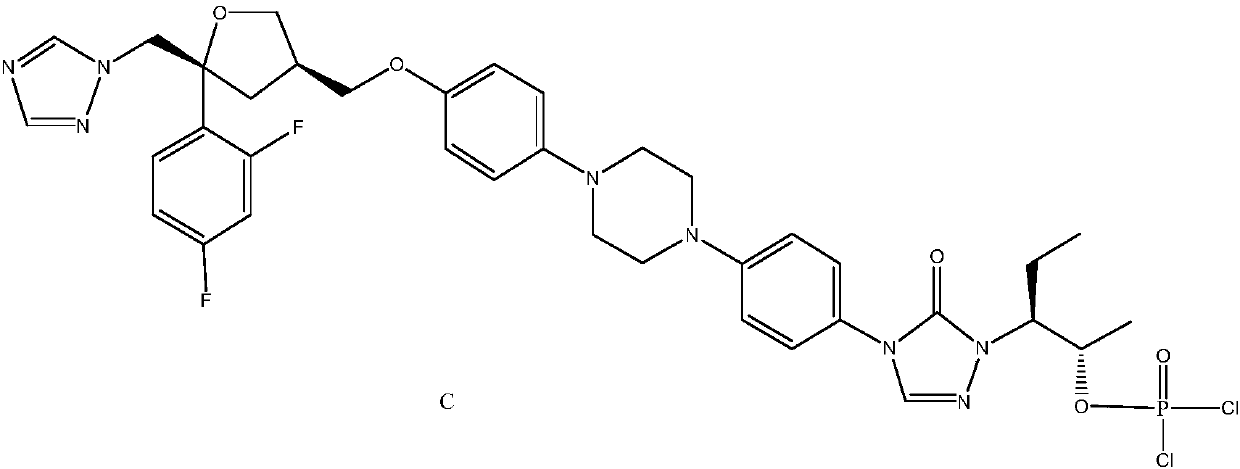
Monocholine salt of posaconazole phosphate, preparation method and applications thereof
A technology of posaconazole phosphate and choline salt, which is applied in the field of posaconazole phosphate monocholine salt and its preparation, can solve the problems of limiting the scope of clinical application of drugs, and achieve improved physical stability and easier handling , Low hygroscopicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of posaconazole phosphate monocholine salt
[0038]
[0039] step 1
[0040]
[0041] Weigh posaconazole (10g, 14.28mmol) into a dry 250mL three-necked flask, add dichloromethane (100mL) under nitrogen protection, stir to dissolve, add triethylamine (5mL) at room temperature (25℃) ) The reaction was stirred at room temperature (25° C.) for 30 min, and phosphorus oxychloride (3 mL, 32.13 mmol) was slowly added, and the addition was completed in about 1 min. The reaction was completed for 6 hours and the reaction was completed. In-process HPLC judges whether the reaction is complete.
[0042] Chromatographic conditions:
[0043] Mobile phase: 6.8g / L potassium dihydrogen phosphate adjust pH to 3.0 with phosphoric acid: acetonitrile=60:40
[0044] Detection wavelength: 220nm Flow rate: 1.0ml / min Column temperature: 25℃
[0045] Sample concentration: 1mg / ml The dilution medium is 50% acetonitrile
[0046] The reaction solution was added dropwise to 150mL pure wa...
Embodiment 2
[0050] Example 2: Preparation of posaconazole phosphate monocholine salt
[0051]
[0052] step 1
[0053]
[0054] Take 40ml of phosphorus oxychloride and place it in a dry 250ml three-necked flask. Under the protection of nitrogen, cool to -5 to 5°C. Slowly add posaconazole (10g, 14.28mmol). After the addition, keep it warm and stir for 12 hours. The reaction is over. In-process HPLC judges whether the reaction is complete.
[0055] Chromatographic conditions:
[0056] Mobile phase: 6.8g / L potassium dihydrogen phosphate adjust pH to 3.0 with phosphoric acid: acetonitrile=60:40
[0057] Detection wavelength: 220nm Flow rate: 1.0ml / min Column temperature: 25℃
[0058] Sample concentration: 1mg / ml The dilution medium is 50% acetonitrile
[0059] The reaction solution was added dropwise to 800 mL of sodium hydroxide aqueous solution at 0°C, and the hydrolysis temperature was controlled to 0-5°C. After the hydrolysis was completed, the pH was adjusted to 3 to 4 with 10% hydrochloric acid....
Embodiment 3
[0063] Example 3: Preparation of posaconazole phosphate monocholine salt pentahydrate
[0064]
[0065] The same batch of posaconazole as in Example 1 was selected to repeat Steps 1 and 2 of Example 2 for the test, except that the drying method of Step 2 was changed to 35±5°C blast drying. The title compound is obtained as a white solid. (Related substances: 0.13%; moisture: 9.06%; content 99.87%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com