Application of isobiflorin or pharmaceutically acceptable salt of isobiflorin from qi-regulating medicine in preparation of antidepressant pharmaceutical composition
An antidepressant and composition technology, applied in the application field of preparing antidepressant pharmaceutical compositions, achieves the effects of high safety, exact curative effect and long history of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The separation of embodiment 1 isobiflorin
[0033] Grind dried cloves (Syzygium aromaticum) into powder, add 70% ethanol at a ratio of 1:10 to liquid, extract twice in a water bath at 60°C for 2 hours each time, and then evaporate the solvent in vacuum. The 70% ethanol extract was suspended in water, and extracted with n-hexane, ethyl acetate and n-butanol in sequence. The n-butanol-soluble fraction was chromatographed on HP-20 and eluted with a water-methanol gradient to obtain several fractions. One of the fractions was further eluted with a water-methanol system (3:7), and then the fraction was recrystallized in methanol to isolate the compound isobiflorin.
Embodiment 2
[0034] Embodiment 2 pharmacodynamics and pharmacology experiment
[0035] 1. Experimental animals: Male ICR mice were purchased from Shanghai Slack Experimental Animal Center, weighing 22-26g, 6 weeks old; the animals were placed in cages of 320×180×160cm, 4 per cage, and the mice were acclimated to the environment for one week; During the whole experiment, the mice were given free access to water and food, the ambient temperature was 22±2°C, the relative humidity was 55±5%, and the light was 12 hours a day.
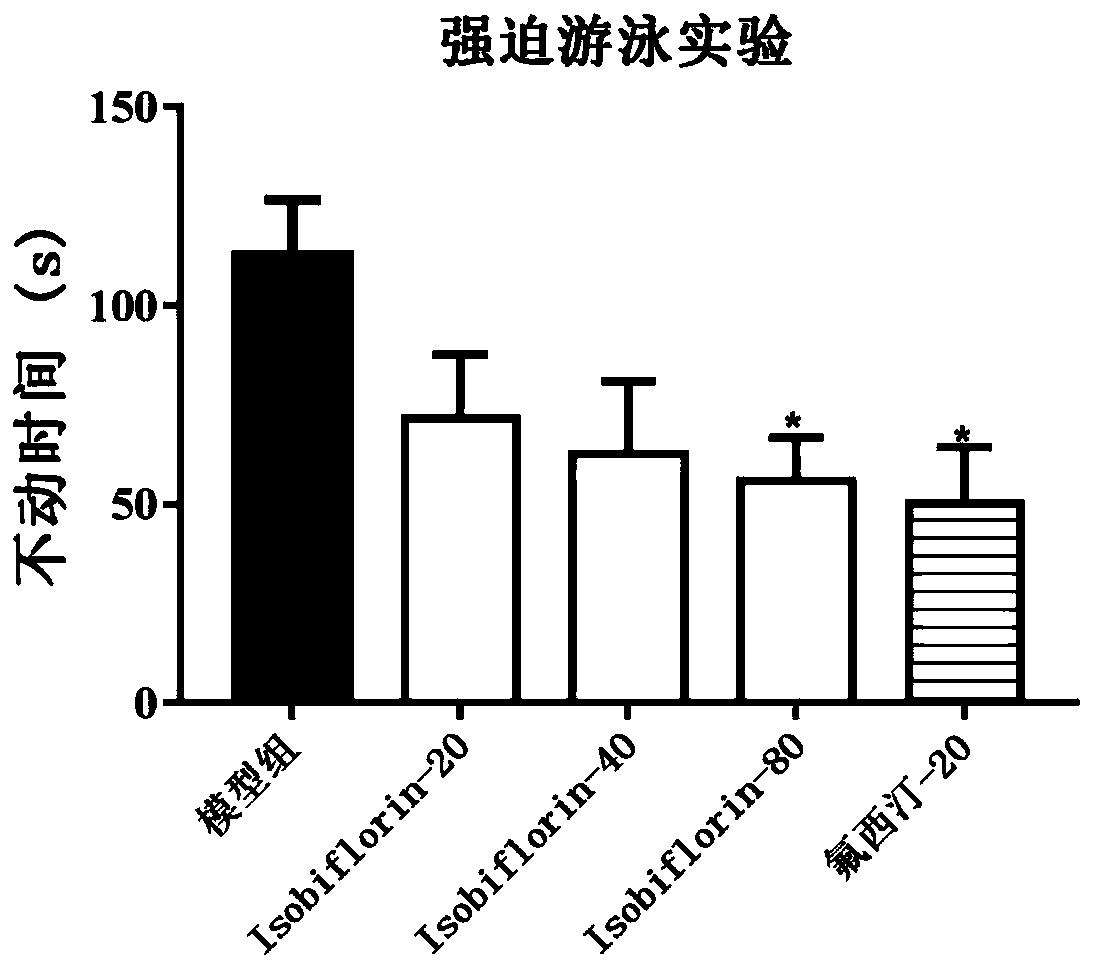
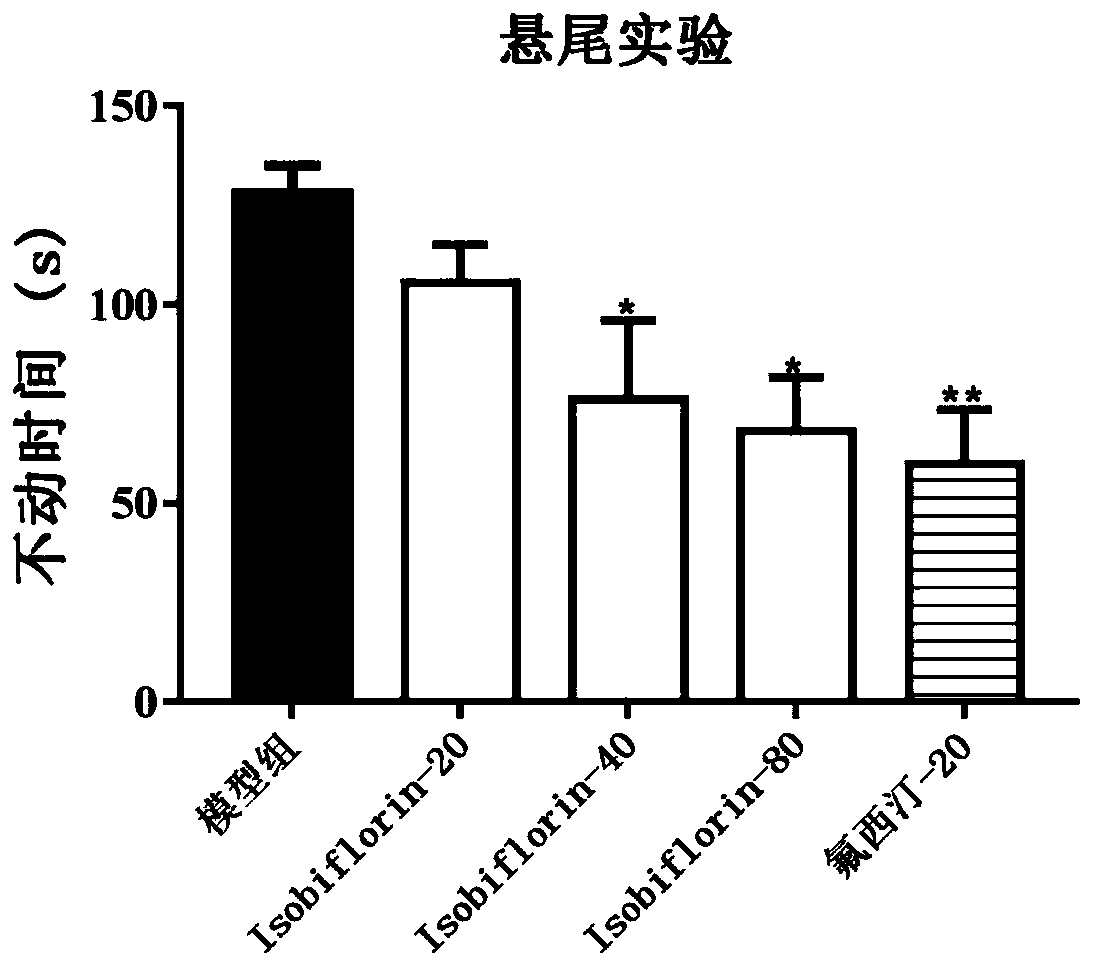
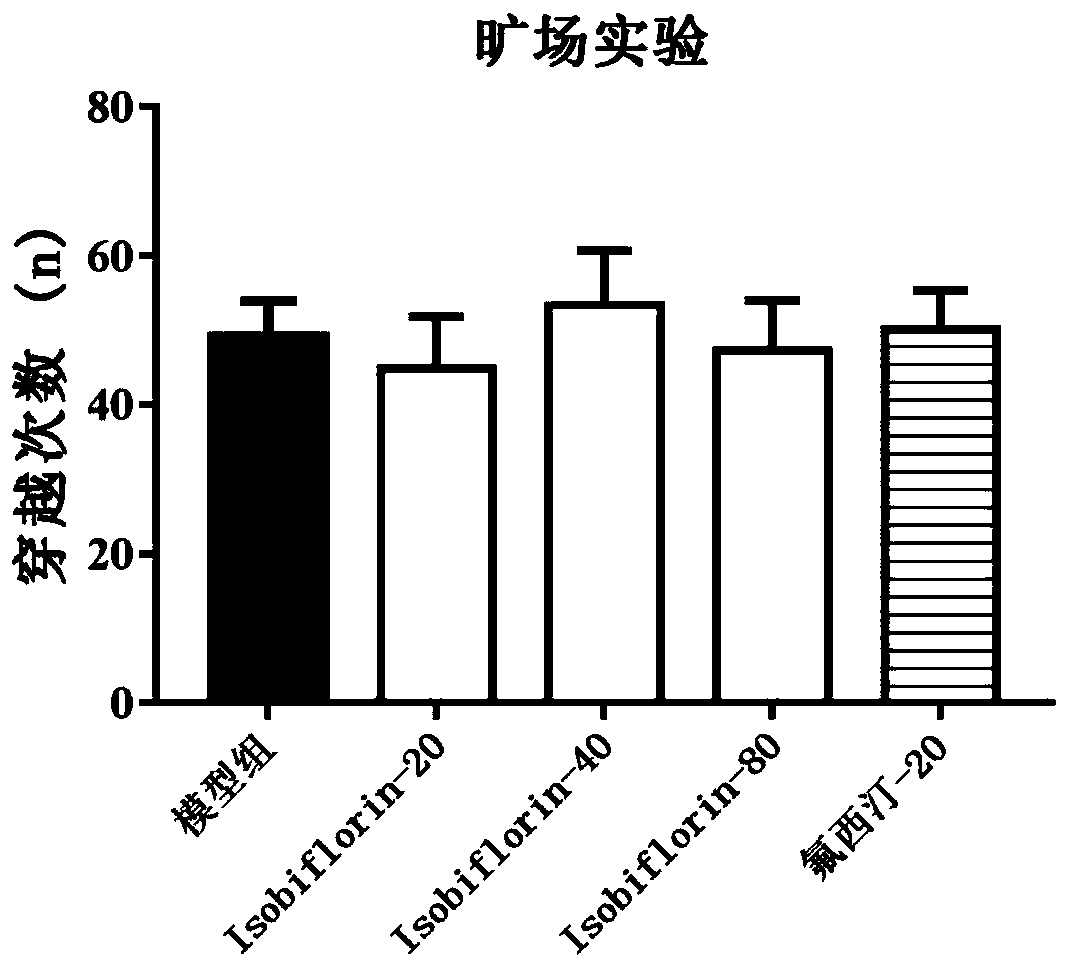
[0036] 2. Dosage: The dosage of Isobiflorin was respectively low-dose group (20mg / kg), middle-dose group (40mg / kg) and high-dose group (80mg / kg). Isobiflorin was administered by intragastric administration, and the positive drug was fluoxetine hydrochloride (20mg / kg). After the administration, the mice will be used for relevant behavioral experiments and determination of biochemical indicators.
[0037] 3. Forced swimming test: Put mice into a cylindrical glass contain...
Embodiment 3
[0048] The preparation of embodiment 3 Isobiflorin tablet
[0049] Prescription (100 capsules): Isobiflorin 0.1g, micronized silica gel 0.5g, lactose 10g, microcrystalline cellulose 10g, sodium carboxymethyl starch 0.05g, magnesium stearate 0.08g, hydroxypropyl methylcellulose 0.1g.
[0050] Preparation method: Mix isobiflorin and micropowder silica gel evenly and micronize; add hydroxypropyl methylcellulose to prepare adhesive; mix lactose, microcrystalline cellulose and sodium carboxymethyl starch; mix the above main ingredients and The excipients are mixed and dried, then magnesium stearate is added to the granules, mixed evenly, and compressed to make 100 tablets with consistent content (each containing 1 mg of isobiflorin). The recommended oral dosage for adults is 1-2 capsules / time, 1-2 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com