Nucleic acid group, kit and detection method for intrauterine microbiological detection of pregnant and lying-in women
A microbial detection and maternal technology, applied in the field of intrauterine microbial detection of pregnant and lying-in women, can solve the problems of low sensitivity, short time-consuming and high false negative rate, so as to improve the detection sensitivity and specificity, improve the sensitivity and specificity, improve the Effects of Specificity and Sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The nucleic acid set provided in this example for detecting intrauterine microorganisms of pregnant and lying-in women includes the following primer pairs, probes and positive oligonucleotide single-stranded DNA:
[0082] The first pair of primers for detecting Group B Streptococcus: it includes the upstream primer shown in SEQ ID NO.3 and the downstream primer shown in SEQ ID NO.4;
[0083] The second pair of primers for detecting Neisseria gonorrhoeae: it includes the upstream primer shown in SEQ ID NO.5 and the downstream primer shown in SEQ ID NO.6;
[0084] The third primer for detecting herpes simplex virus type II: it includes the upstream primer shown in SEQ ID NO.7 and the downstream primer shown in SEQ ID NO.8;
[0085] The fourth primer pair for detecting human cytomegalovirus: it includes the upstream primer shown in SEQ ID NO.9 and the downstream primer shown in SEQ ID NO.10;
[0086] The fifth primer pair for detecting Chlamydia trachomatis: it includes t...
Embodiment 2
[0107] This embodiment provides a method for detecting microorganisms in the uterus of pregnant and lying-in women using the nucleic acid group of Embodiment 1, and the operation is as follows:
[0108] (1) Extract the genomic DNA of the sample to be tested
[0109] The genital secretions were collected with a cotton swab test, and the total nucleic acid of the sample was extracted with a bacterial / viral DNA extraction kit for later use.
[0110] The concentration and quality of the extracted total nucleic acid were measured by an ultra-micro spectrophotometer (NanoDrop 2000), and then multiplex PCR was performed.
[0111] (2) Multiplex PCR system and conditions
[0112] 2×Multiplex PCR Master Mix (containing UNG enzyme): 10μL;
[0113] Primers: the final concentration is 0.2 μM (upstream primer) / 0.3 μM (downstream primer);
[0114] Extracted sample genomic DNA: 2-100ng;
[0115] Internal control, human β-actin gene fragment recombinant plasmid DNA: 2ng;
[0116] Positive...
Embodiment 3
[0144] (1) Specific detection
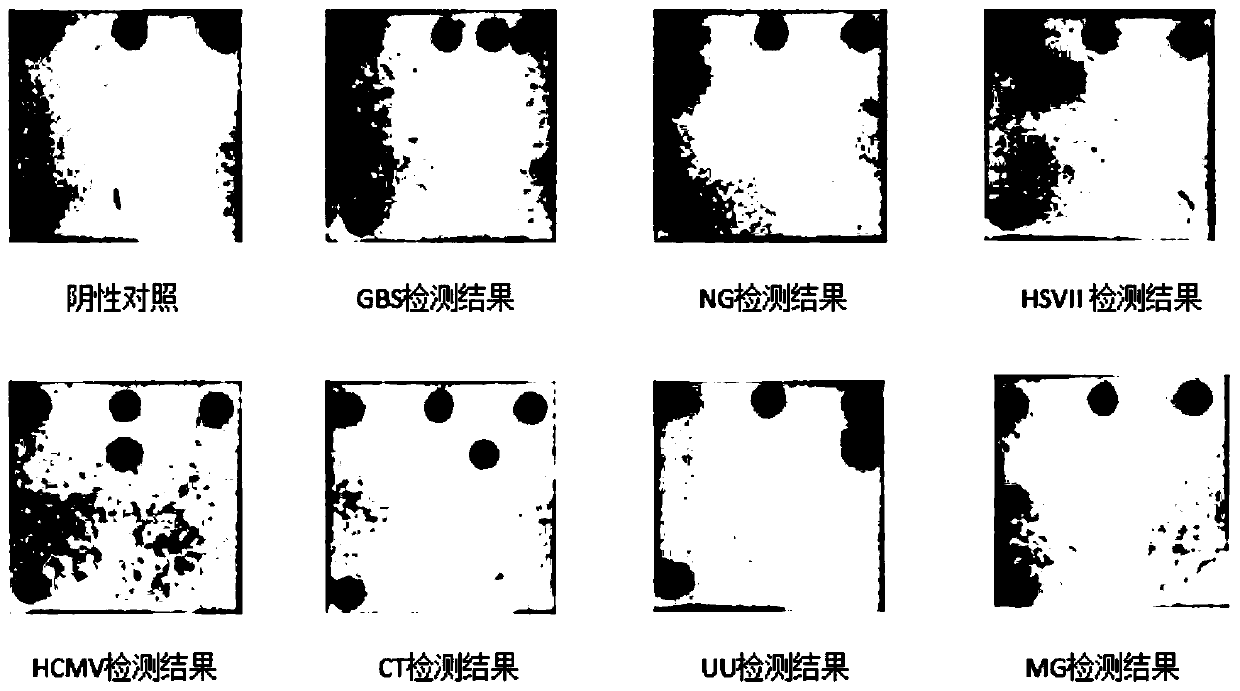
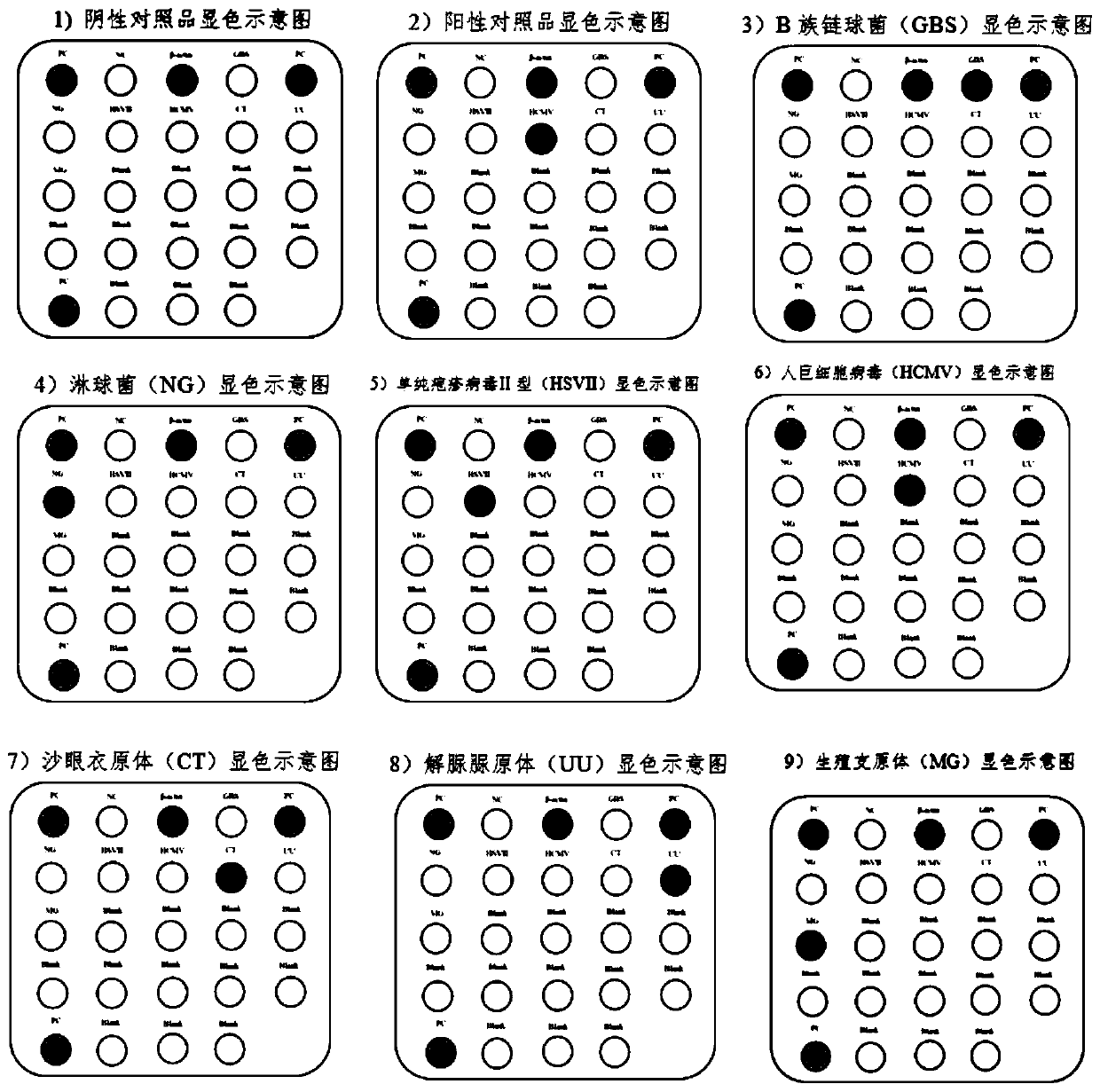
[0145] The total nucleic acids of seven microorganisms, GBS, NG, HSVII, HCMV, CT, UU, and MG, were extracted using commercial bacterial / viral DNA extraction kits as test objects. At the same time, human genome DNA was extracted as a control, and the internal control was recombinant plasmid DNA of human β-actin gene fragment, and the negative control was extracted human whole genome DNA not infected with the seven microorganisms. The extracted DNA was amplified by PCR after its concentration and quality were measured with an ultramicro spectrophotometer (NanoDrop 2000).
[0146] Adopt the nucleic acid group of embodiment 1, and detect according to the method for embodiment 2, the result is as follows figure 2 shown.
[0147] The negative control contains human genomic DNA and does not contain any microbial DNA within the detection range of this method. Therefore, except for β-actin and PC, other spots should not develop color. The actual test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com