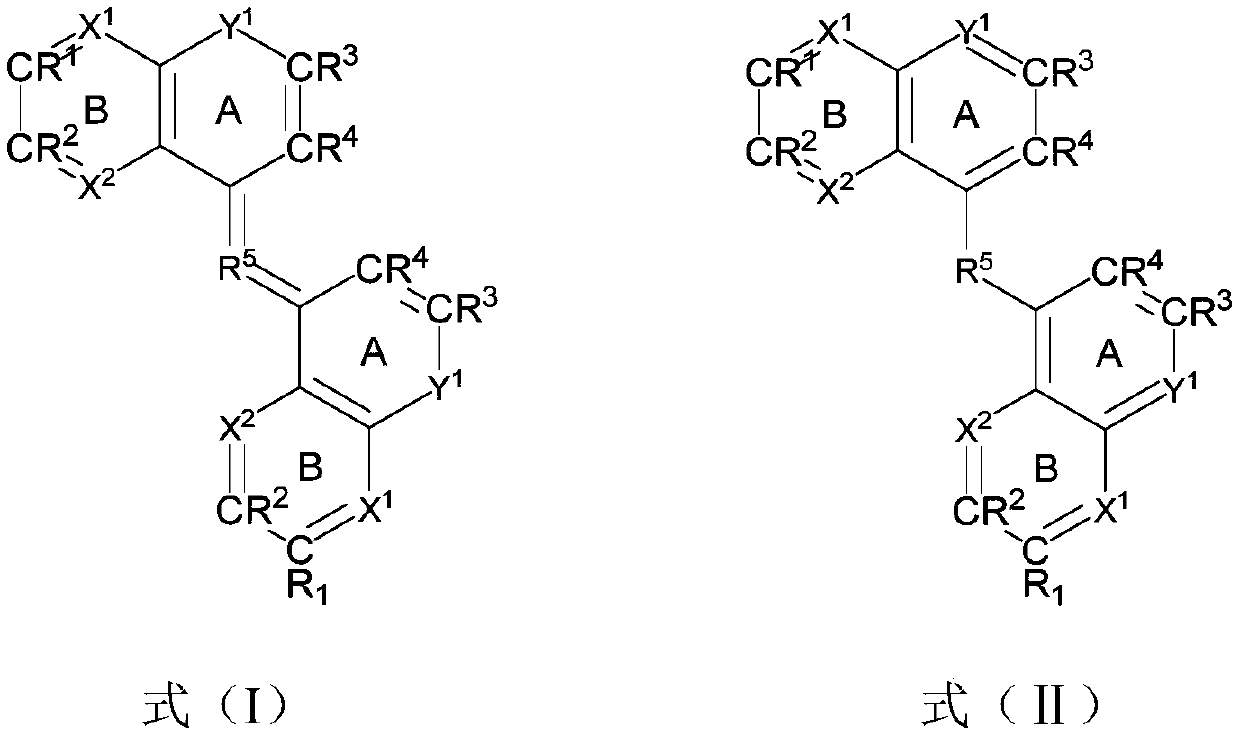
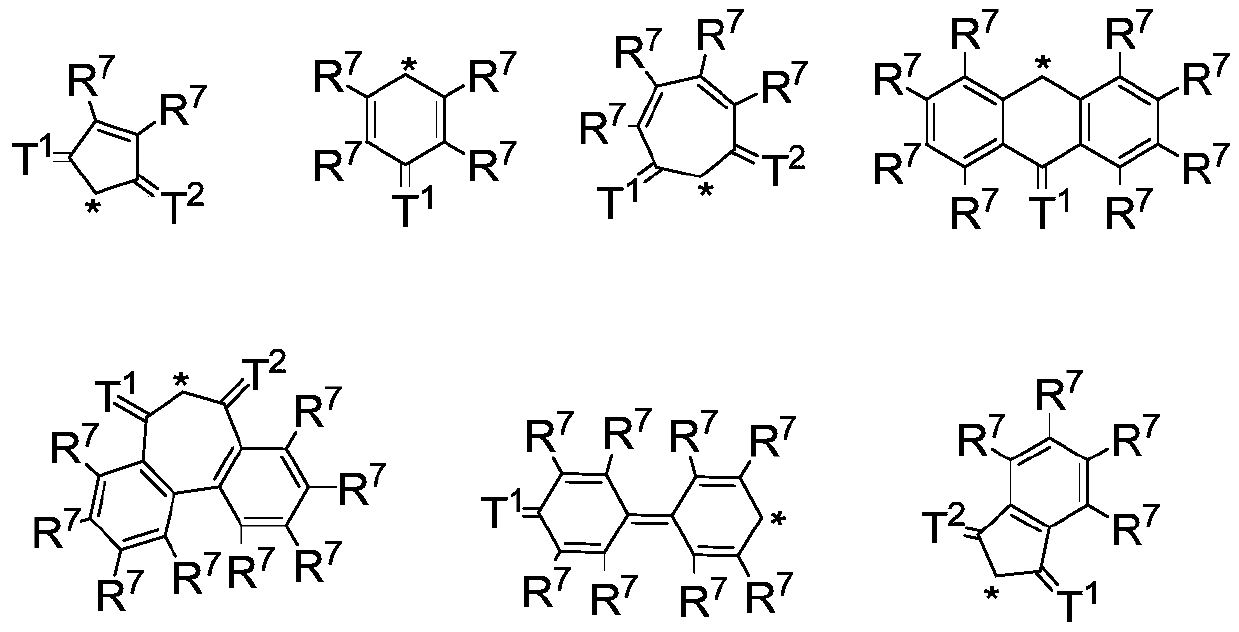
Condensed-ring aryl compound, organic electronic device, and application thereof
A technology of fused-ring aromatic groups and compounds, which is applied in the field of optoelectronic materials and can solve the problems of short lifespan of P-doped materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] Synthesis of intermediate 1-P-1: In a 250 ml three-necked flask, add 4-bromo-2,3,5,6-tetrafluorobenzonitrile (25.29 g, 1 equivalent, 0.1 mol) under nitrogen protection, Tetrahydrofuran (80 milliliters), under the condition of -78 degrees Celsius, slowly add n-butyl lithium (0.1mol) dropwise. :1-5:1), the intermediate 1-P-1 (13.22 g, yield: 38%) was obtained.
[0051] Synthesis of P-1: In a 100 ml three-necked flask, under nitrogen protection, add intermediate 1-P-1 (3.48 g, 1 equivalent, 0.01 mol), ethanol (30 ml), 2,3-diaminocis-butyl Dicyandiamide (4.32 g), slowly added sodium tert-butoxide (8 equivalents), refluxed for 12 hours, after the reaction was completed, cooled to room temperature, quenched with 10 ml of ice water, extracted with dichloromethane, the concentrate was rotary evaporated, beaten (n-hexane: dichloromethane: acetonitrile = 10:3:2) three times, acetonitrile recrystallization once to obtain P-1 (2.57 g, yield 42%).
[0052] Elemental An...
Embodiment 2
[0054]
[0055] The synthesis of intermediate 1-P-2: the same as the synthesis of intermediate 1-P-1, the difference is that 2-bromo-3,4,5,6-tetrafluorobenzonitrile is used instead of 4-bromo-2,3, 5,6-tetrafluorobenzonitrile to obtain intermediate 1-P-2 (11.83 g, yield 34%).
[0056] Synthesis of P-2: Same as the synthesis of P-1, except that intermediate 1-P-2 was used instead of intermediate 1-P-1 to obtain P-2 (2.75 g, yield 45%).
[0057] Elemental Analysis: C 30 N 18 Theoretical: C, 58.83; N, 41.17; Found: C, 58.80; N, 41.20; HRMS (ESI) m / z(M): Theoretical: 612.0553; Found: 612.0560.
Embodiment 3
[0059]
[0060]The synthesis of intermediate 1-P-3: the synthesis of intermediate 1-P-1, the difference is that 1-bromo-2,3,5,6-tetrafluoro-4-nitrobenzene (27.2 grams, 1 equivalent , 0.1mol) instead of 4-bromo-2,3,5,6-tetrafluorobenzonitrile to obtain intermediate 1-P-3 (17.8 g, yield 46%).
[0061] The synthesis of intermediate 2-P-3: the same as the synthesis of P-1, the difference is that intermediate 1-P-3 (3.9 g, 1 equivalent) is used instead of intermediate 1-P-1 to obtain intermediate 2-P -3 (3.0 g, 46% yield).
[0062] Synthesis of P-3: In a 50 ml three-neck flask, under nitrogen protection, add intermediate 2-P-3 (6.5 g, 1 equivalent, 0.01 mol), 4,7,13,16,21,24-hexaoxo -1,10-diazabicyclo[8.8.8]hexacane (0.01mol), DMF (15 milliliters), stirred at 150°C for 1 hour, cooled to room temperature after the reaction was completed, added a mixture of water and dichloromethane ( 5:3), the product entered the dichloromethane layer, and after separation, (n-hexane:dichlorome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com