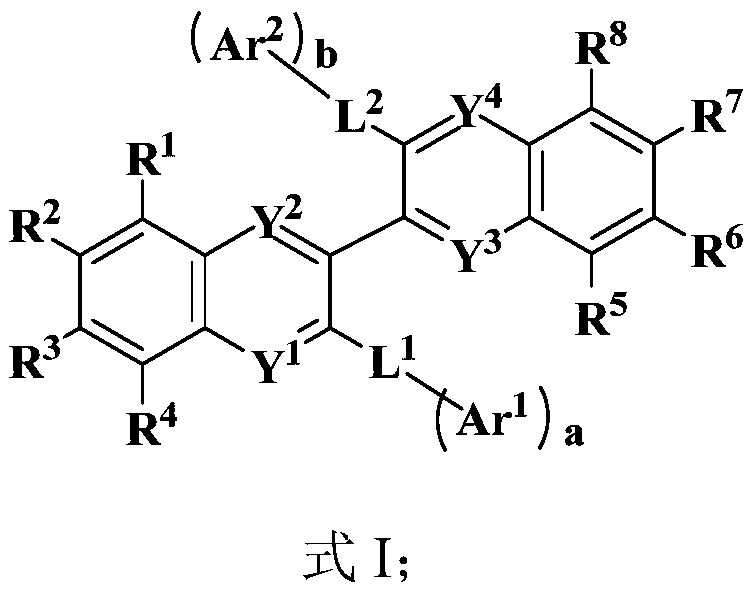
Organic electroluminescent compound, preparation method and application thereof
An electroluminescence and compound technology, which is applied in the field of organic electroluminescence compounds and their preparation, can solve the problems of high driving voltage and need to improve current efficiency, and achieves the advantages of improving device efficiency, preventing molecular crystallization of light-emitting layer materials, and improving color purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0103] This synthesis example provides an intermediate 1 and its synthesis route:
[0104]
[0105] In a 1L three-necked flask at -78°C under nitrogen, add a solution of 2,2,6,6-tetramethylpiperidine (TEMPO, 20mL) in anhydrous tetrahydrofuran (200mL), and add Butyllithium (n-BuLi, 160mmol) tetrahydrofuran solution (80mL), reacted at 0°C for 20 min, put the reaction solution back at -78°C, and added dropwise starting material 1: 2-chloroquinoxaline (16.5g , 100mmol) in tetrahydrofuran (100mL), react at low temperature for 30min, add 200mL concentrated hydrochloric acid and 300mL ethanol dropwise to the reaction solution, and stir for 1h. The reaction solution was extracted with dichloromethane and purified by column chromatography (mixed solvent of petroleum ether PE and ethyl acetate EA) to obtain intermediate 1: 3,3'-dichloro-2,2'-biquinoxaline ( 19.2 g, 59% yield).
[0106] Intermediate 1 was prepared according to the above synthetic route, and the specific structural f...
Synthetic example 2
[0112] This synthesis example provides a raw material And its synthetic route:
[0113]
[0114]In a dry 500mL double-neck round-bottom flask, fill nitrogen, add 2-naphthyl bromide (20.6g, 0.1mol), double pinacol base diboron (25.4g, 0.1mol), potassium acetate (19.6g, 0.2 mol), [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.005mol), 1,4-dioxane (200mL), react at 120°C for 8h; After the reaction is complete, cool to room temperature, quench with water, extract with dichloromethane (200mL extraction three times), the extract is dried with anhydrous magnesium sulfate, spin-dried, and the crude product is separated by silica gel column chromatography to obtain 2-naphthylboronic acid Nacohol ester (15.5 g, 61% yield).
Synthetic example 3
[0116] The difference between this synthesis example and synthesis example 2 is that 2-naphthyl bromide is used with an equimolar amount of 3-bromo-1,1'-biphenyl Substitute to get 3-biphenylboronic acid pinacol ester (17.9 g, 64% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com