Flavonoid glycoside adsorption resin and preparation method thereof
A technology for adsorbing resin and flavonoid glycosides, applied in chemical instruments and methods, other chemical processes, alkali metal oxides/hydroxides, etc., can solve the problems of complex separation operation process, low separation efficiency, poor specificity, etc. The operation process is simple, the separation efficiency is high, and the elution is easy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
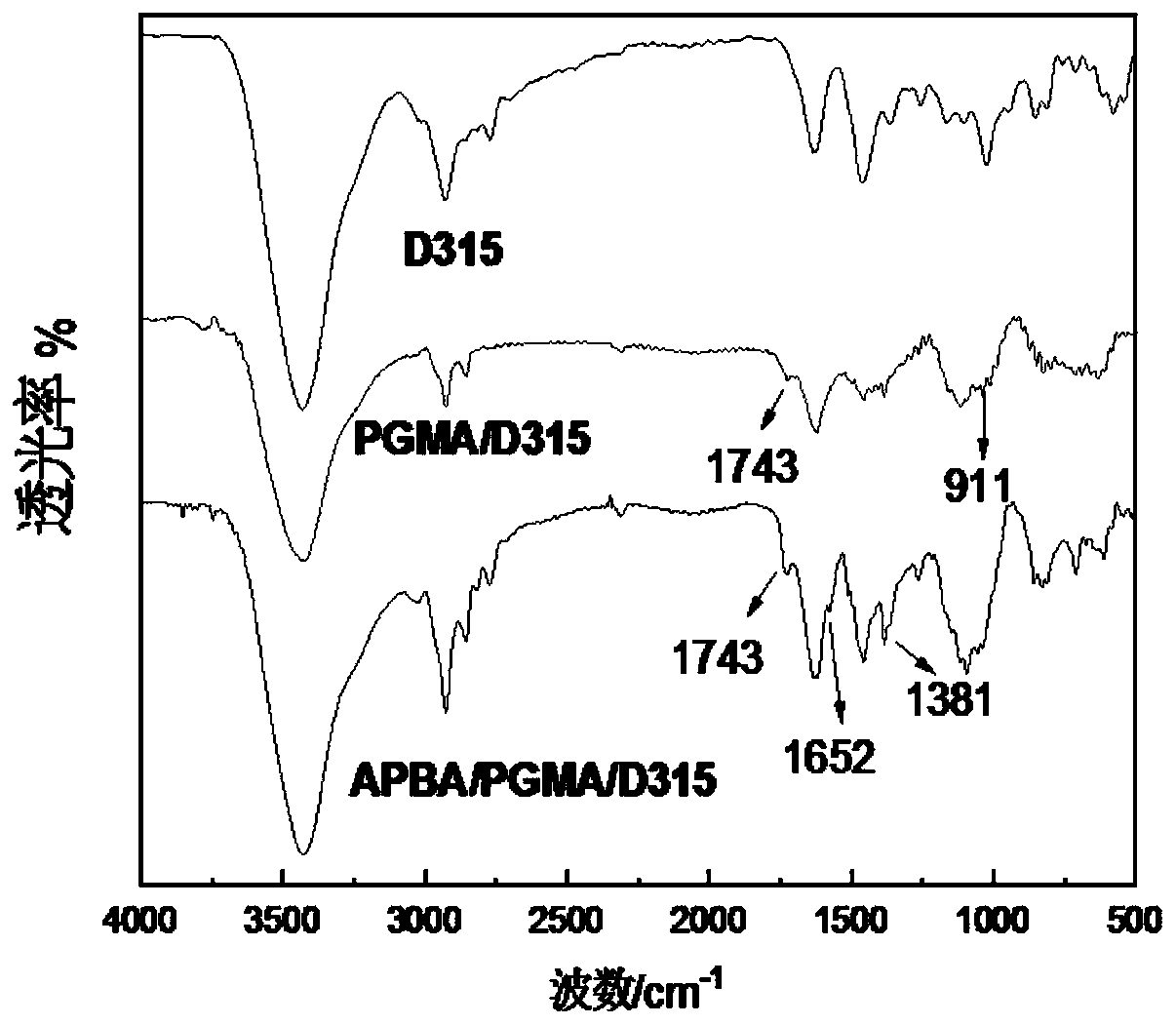
[0033] The macroporous resin D941 with primary amino groups is used as the matrix.
[0034] Step 1: Pretreatment of macroporous resin D941
[0035] Soak the macroporous resin D941 in deionized water overnight, then wash it repeatedly with distilled water, and finally filter it with suction and dry it for later use.
[0036] Step 2: Grafting organic long-chain polymers on the surface of macroporous resin D941
[0037] Glycidyl methacrylate (GMA) is used as a functional monomer to graft an organic long-chain polymer on the surface of the macroporous resin D941 through a free radical chain reaction to obtain the grafted material pGMA / D941, specifically:
[0038] Put 1g of pretreated macroporous resin D941, 100mL of N,N-dimethylformamide (DMF) and 15mL of functional monomer GMA into a 250mL four-necked flask, and use N 2 Empty the air in the four-necked flask, then raise the temperature of the water bath to 60°C, add 0.10g of initiator ammonium persulfate, and carry out graft po...
Embodiment 2
[0045] The macroporous resin D315 with primary amino groups is used as the matrix.
[0046] Step 1: Pretreatment of macroporous resin D315
[0047] Soak the macroporous resin D315 in deionized water overnight, then wash it repeatedly with distilled water, and finally filter it with suction and dry it for later use.
[0048] Step 2: Grafting organic long-chain polymers on the surface of macroporous resin D315
[0049] Using GMA as a functional monomer, graft organic long-chain polymers on the surface of macroporous resin D315 through free radical chain reaction to obtain grafted material pGMA / D315, specifically:
[0050] Put 1g of pretreated macroporous resin D315, 100mL DMF and 15mL functional monomer GMA into a 250mL four-necked flask, and use N 2Evacuate the air in the four-necked flask, then raise the temperature of the water bath to 50°C, add 0.15g of initiator ammonium persulfate, and carry out graft polymerization reaction under constant temperature and stirring condit...
Embodiment 3
[0058] The macroporous resin D315 with primary amino groups is used as the matrix.
[0059] Step 1: Pretreatment of macroporous resin D315
[0060] The same method as that of Embodiment 2 is adopted, and details are not repeated here.
[0061] Step 2: Grafting organic long-chain polymers on the surface of macroporous resin D315
[0062] Put 1g of pretreated macroporous resin D315, 100mL DMF and 20mL functional monomer GMA into a 250mL four-necked flask, and use N 2 Empty the air in the four-necked flask, then raise the temperature of the water bath to 55°C, add 0.20g of initiator ammonium persulfate, and carry out graft polymerization reaction under constant temperature and stirring conditions, the reaction time is 18h, and then suction filtration, The filter cake was washed with absolute ethanol to remove the polymer physically adsorbed on the surface of the microspheres to obtain the grafted material pGMA / D315, dried in vacuum and set aside.
[0063] Step 3: Incorporation...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com