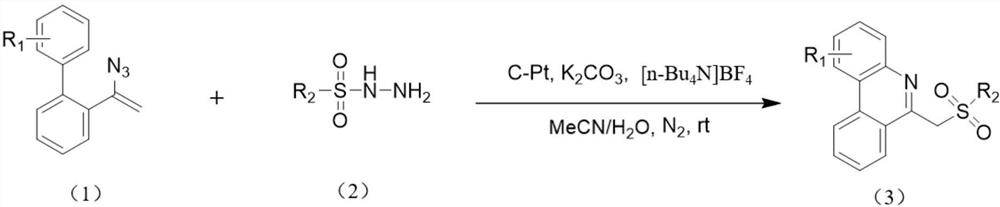
A kind of electrocatalytic preparation method of 6-(sulfonylmethyl)phenanthridine compounds
A technology of sulfonylmethyl compounds, which is applied in the field of electrocatalytic preparation of 6-phenanthridine compounds, can solve the problems of reducing the use of organic solvents, easy pollution of the environment, and the expensive price of terpyridylruthenium chloride hexahydrate photosensitizers. , to achieve the effect of simple operation steps, mild conditions and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 6-(tosylmethyl)phenanthridine:
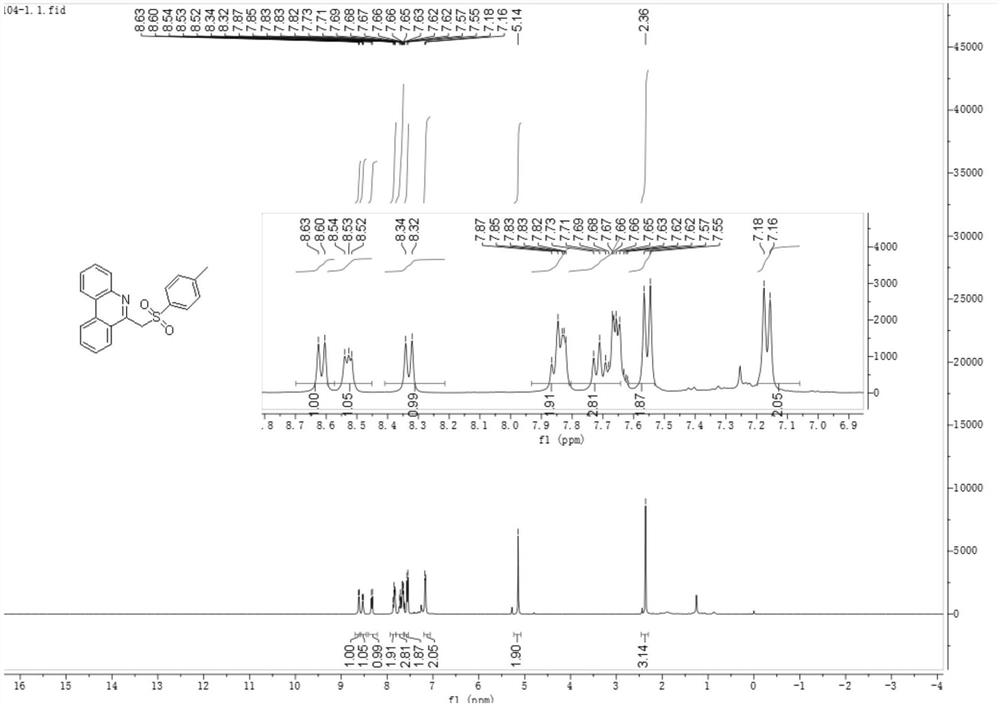
[0038] Under nitrogen, add tetrabutylammonium tetrafluoroborate (26.3 mg, 0.08 mmol), anhydrous potassium carbonate (41.4 mg, 0.3 mmol), 4-methylbenzenesulfonyl hydrazide (55.9 mg , 0.3mmol), distyryl azide (44.2mg, 0.2mmol), with 4mL of acetonitrile and water mixed solvent (acetonitrile and water volume ratio is 18:1) to dissolve the above substances, use carbon electrode as working electrode, platinum sheet The electrode was a counter electrode, and the reaction mixture was reacted for 3 hours under the condition of a constant current of 12 mA. After the reaction was completed, the aqueous phase was extracted with dichloromethane, the organic phase was dried with anhydrous sodium sulfate, and the reaction solution after separation, extraction, and drying by column chromatography obtained 59.68 mg of a white solid, namely 6-(tosylmethyl) Phenanthridine 59.68 mg, the calculated yield was 86%. The product...
Embodiment 2
[0046] Embodiment 2: the influence of different acetonitrile and water volume ratio on the preparation of 6-(tosylmethyl)phenanthridine
[0047] On the basis of Example 1, further explore the impact of different acetonitrile and water volume ratios on the preparation of 6-(tosylmethyl)phenanthridine, acetonitrile and water according to the volume ratio of 1:1, 10:1, 15:1 , 18:1, and 20:1 were mixed, and other reaction conditions were the same as in Example 1 to prepare 6-(tosylmethyl)phenanthridine respectively, and the results are shown in Table 1. As can be seen from Table 1, when the volume ratio of acetonitrile and water was 1:1, the product quality was only 13.88mg, and the yield was only 20%. When gradually increasing the volume ratio of acetonitrile and water, the quality and yield of the product also increased. Gradually increased, when the volume ratio of acetonitrile and water was 18:1, the product quality reached 59.68 mg, and the yield reached 86%. Continuing to i...
Embodiment 3
[0050] Embodiment 3: the impact of different electrocatalytic currents on the preparation of 6-(tosylmethyl)phenanthridine
[0051] On the basis of Example 1, the influence of different electrocatalytic currents on the preparation of 6-azidomethylphenanthridine was further explored. The energizing current was set to 6mA, 8mA, 10mA, 12mA, 16mA, 20mA, and other reaction conditions were the same as in Example 1 to prepare 6-azidomethylphenanthridine respectively. The results are shown in Table 2. As can be seen from Table 2, when the electrocatalytic current is 6mA, the product quality is 5.55mg, and the yield is only 8%. When the electrocatalytic current is gradually increased, the quality and yield of the product are also gradually improved. When the electrocatalytic current increases to At 12mA, the product quality reached 59.68mg, and the yield reached 86%. When the electrocatalytic current was further increased, the quality and yield of the product began to decrease again. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resonant frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com