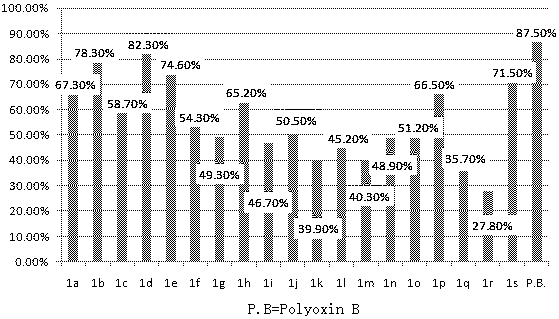
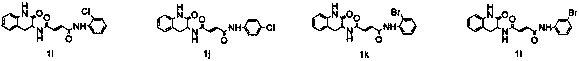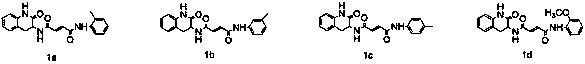Design, synthesis and application of quinolinone fumaramide derivatives
A kind of technology of phenyl fumarate and tetrahydroquinoline, applied in the field of chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of aniline chalcone 3a:
[0028] Maleic anhydride (2.37g, 24.16mmol) was dissolved in 60ml of anhydrous dichloromethane, aniline (2ml, 21.96mmol) was added, and the mixture was stirred at room temperature for 2 hours, a white precipitate was formed, filtered and distilled Wash with methyl chloride to obtain a pure solid, white powder; the synthesis method of compound 3b-s is the same as that in Example 1.
Embodiment 2
[0029] Example 2: N 1 -(2-oxo-1,2,3,4-tetrahydroquinolin-3-yl)-N 4 -Synthesis of (o-tolyl)fumaramide) (1a)
[0030] Add CH to a 50 mL round bottom flask 2 Cl 2 (30 mL), add triethyl phosphite (0.44ml, 2.5 mmol) under ice-water bath, I 2 (0.636g, 2.5 mmol), add acid (0.510g, 2.5mmol) and triethylamine (1.74ml, 12.5mmol) after the iodine element is completely dissolved, after stirring for 20 min, add compound amine (0.487g, 3.0mmol) dropwise , remove the ice-water bath, react at room temperature for 3 hours, and detect the reaction by TLC spot plate. After the reaction is completed, remove the solvent by rotary evaporation, add 50ml ethyl acetate, wash with 1M HCl (30ml×3), and then wash with saturated sodium bicarbonate solution (30ml ×3), using anhydrous sodium sulfate to remove water, and rotary evaporation to remove the solvent to obtain a white solid, which was recrystallized with methanol / ethyl acetate to obtain the pure target compound with a yield of 80%. The phys...
Embodiment 3
[0031] Example 3: N 1 -(2-oxo-1,2,3,4-tetrahydroquinolin-3-yl)-N 4 -Synthesis of (m-tolyl)fumaramide (1b)
[0032] The synthetic method of compound 1b is the same as above, and the physical constants and spectral data of 1b are as follows:
[0033] 1 H NMR (400 MHz, DMSO-d 6 ) δ 11.01 (s, 1H CONH ), 10.41 (s, 1H CONH ),9.12 (s, 1H CONH ), 7.47-7.38 (m, 2H Ar-H ), 7.25-7.14 (m, 3H Ar-H), 6.95 (t, J =7.4 Hz, 1H Ar-H ), 6.89 (d, J = 7.7 Hz, 2H Ar-H ), 6.37 (s, 2H CH=CH), 4.62- 4.50 (m, 1H quinoline-CH ), 3.18-3.08 (m, 1H), 2.97-2.86 (m, 1H), 2.28 (s,3H Ar-CH 3 ). 13 C NMR (101 MHz, DMSO-d 6 ) δ 168.64, 165.06, 163.79, 139.16,138.42, 137.90, 134.30,130.42, 129.07, 128.63, 128.07, 124.76,122.79, 122.70,120.34, 117.09, 115.63, 48.63, 31.66, 21.64. HRMS (ESI): calcd for C 20 h 19 N 3 NaO 3 [M+Na] + , 372.1319, found, 372.1318.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com