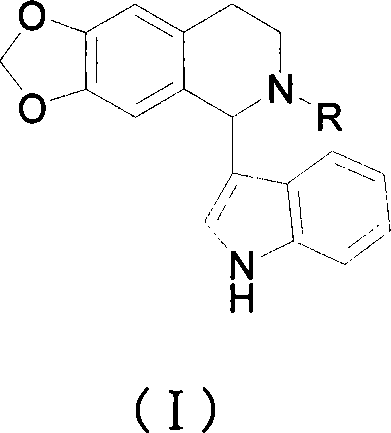
1-(3-indolyl)-6,7-methylene dioxy-1,2,3,4-tetrahydro isoquinoline derivative and its prepn and use
A methylenedioxy, tetrahydroisoquinoline technology, applied in botanical equipment and methods, chemicals for biological control, biocides, etc., can solve multidrug resistance, rapid tumor cell growth, Variability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Synthetic compound 1,1-(3-indolyl)-2-benzoyl-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline
[0098] 1) Preparation of 1,2-methylenedioxy-4-(2-nitro-vinyl)-benzene
[0099] Put 17.5g of piperonal (0.117mol) and 7.63g (0.096mol) of ammonium acetate into 80ml of glacial acetic acid, add 51.8ml (0.924mol) of nitromethane, heat and reflux for 2 hours under the protection of nitrogen flow, and put it in the refrigerator after cooling Leave to stand for 4 hours, filter with suction, wash the filter cake with a small amount of cold ethanol, drain the solvent, and dry under an infrared lamp. 18 g of pale yellow needle-like solid were obtained, yield: 80.0%. Melting point: 161-163°C.
[0100] 2) Preparation of 2-(1,2-methylenedioxy-4-phenyl)-ethylamine hydrochloride
[0101] 4.8g HgCl 2 Dissolve in 96ml of water, add 2.4ml of concentrated hydrochloric acid, add 60g of zinc powder under rapid stirring, and stir for 20 minutes. Suction filtration to obtain zinc-...
Embodiment 2
[0115] Example 2: Synthetic compound 2,1-(3-indolyl)-2-o-chlorobenzoyl-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline
[0116] 1-(3-indolyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline reacts with o-chlorobenzoyl chloride, and the synthesis process is the same as 1. Yield: 36.2%. Melting point: 150-154°C. 1 HNMR (CDCl 3 )δ (ppm): 2.60 (m, 1H, Isoquinoline-C 4 -H), 3.00(m, 1H, Isoquinoline-C 4 -H), 3.35(m, 1H, Isoquinoline-C 3 -H), 3.40(m, 1H, Isoquinoline-C 3 -H), 5.94(d, 2H, OCH 2 O, J = 5.8 Hz), 6.62 (s, 1H, Isoquinoline-C 8 -H), 6.67(s, 1H, Isoquinoline-C 5 -H), 6.80(d, 1H, Isoquinoline-C 1 -H, J=6.4Hz), 7.02-7.42(m, 8H, Ar-H), 7.84(t, 1H, Indole-C 4’ -H, J=7.8 Hz), 8.22 (d, 1H, Indole-NH, J=8.3 Hz). MS (ESI) m / z (M-1) 429.1.
Embodiment 3
[0117] Example 3: Synthetic compound 3,1-(3-indolyl)-2-p-chlorobenzoyl-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline
[0118] 1-(3-indolyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline reacts with p-chlorobenzoyl chloride, the synthesis process is the same as 1. Yield: 45.0%. Melting point: 220-222°C. 1 HNMR (CDCl 3 ) δ (ppm): 2.65 (m, 1H, Isoquinoline-C 4 -H), 2.90(m, 1H, Isoquinoline-C 4 -H), 3.43(m, 1H, Isoquinoline-C 3 -H), 3.54 (m, 1 H, Isoquinoline-C 3 -H), 5.94(d, 2H, OCH 2 O, J=5.8 Hz), 6.64 (s, 1H, Isoquinoline-C 8 -H), 6.66(s, 1H, Isoquinoline-C 5 -H), 6.78(s, 1H, Isoquinoline-C 1 -H), 7.08-7.46(m, 8H, Ar-H), 7.78(d, 1H, Indole-C 4’ -H, J=7.7 Hz), 8.37 (br-s, 1H, Indole-NH). MS (ESI) m / z (M+1) 431.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com