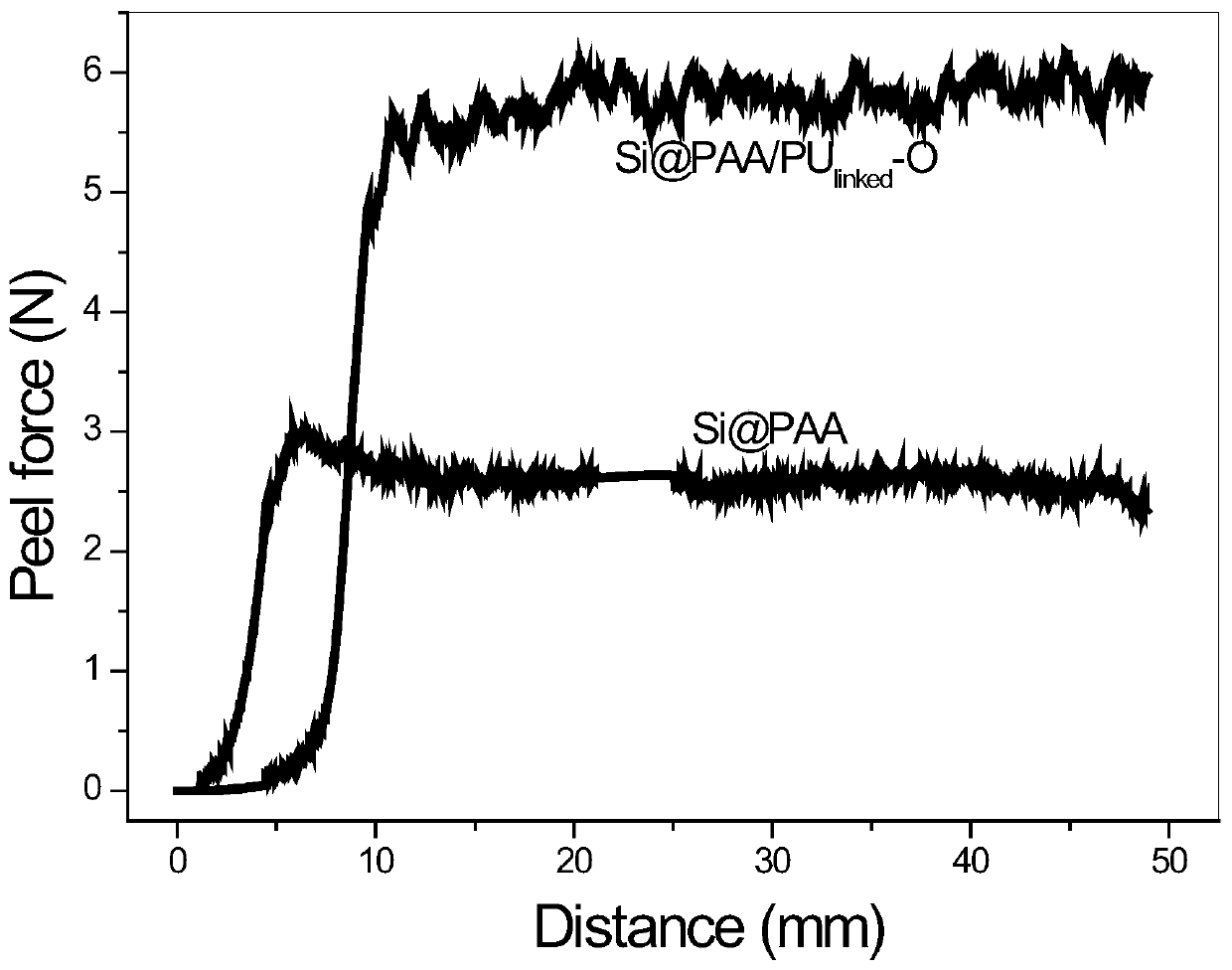
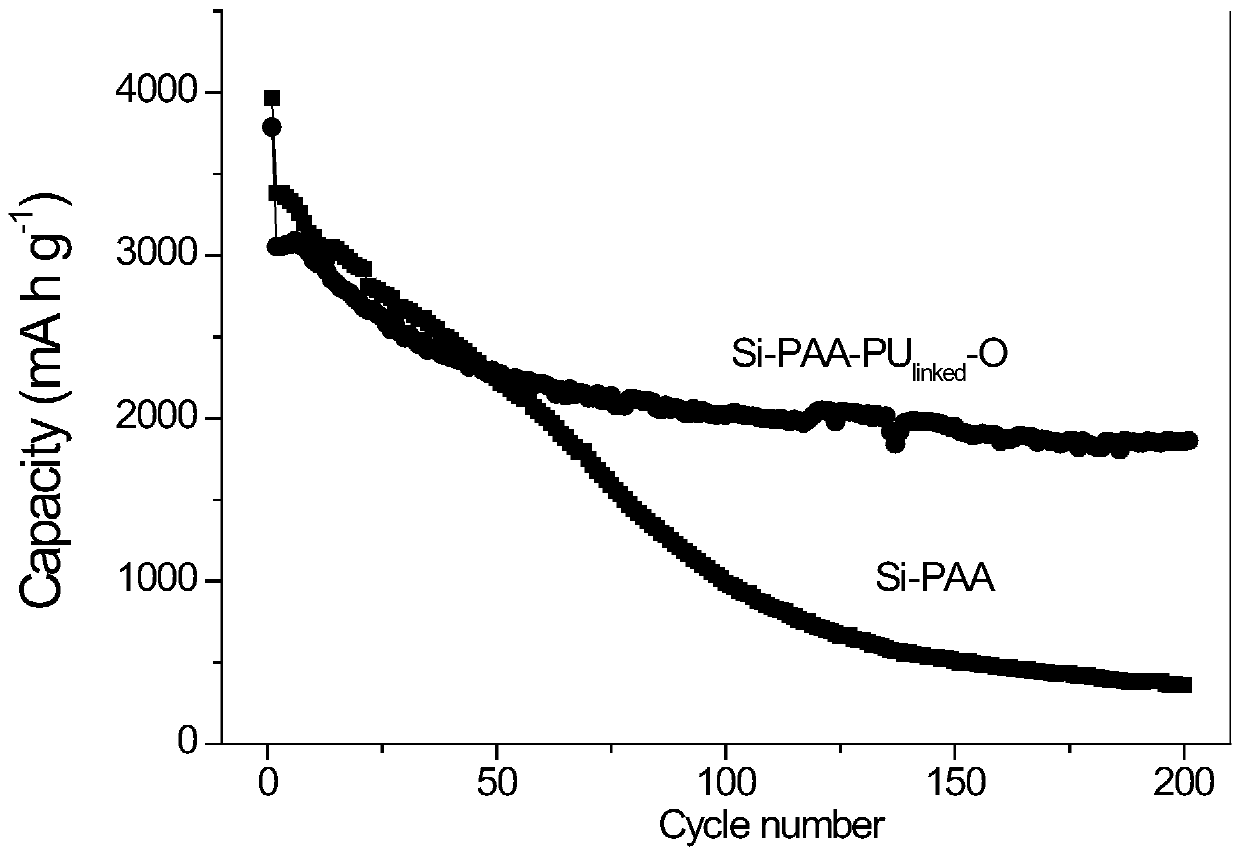
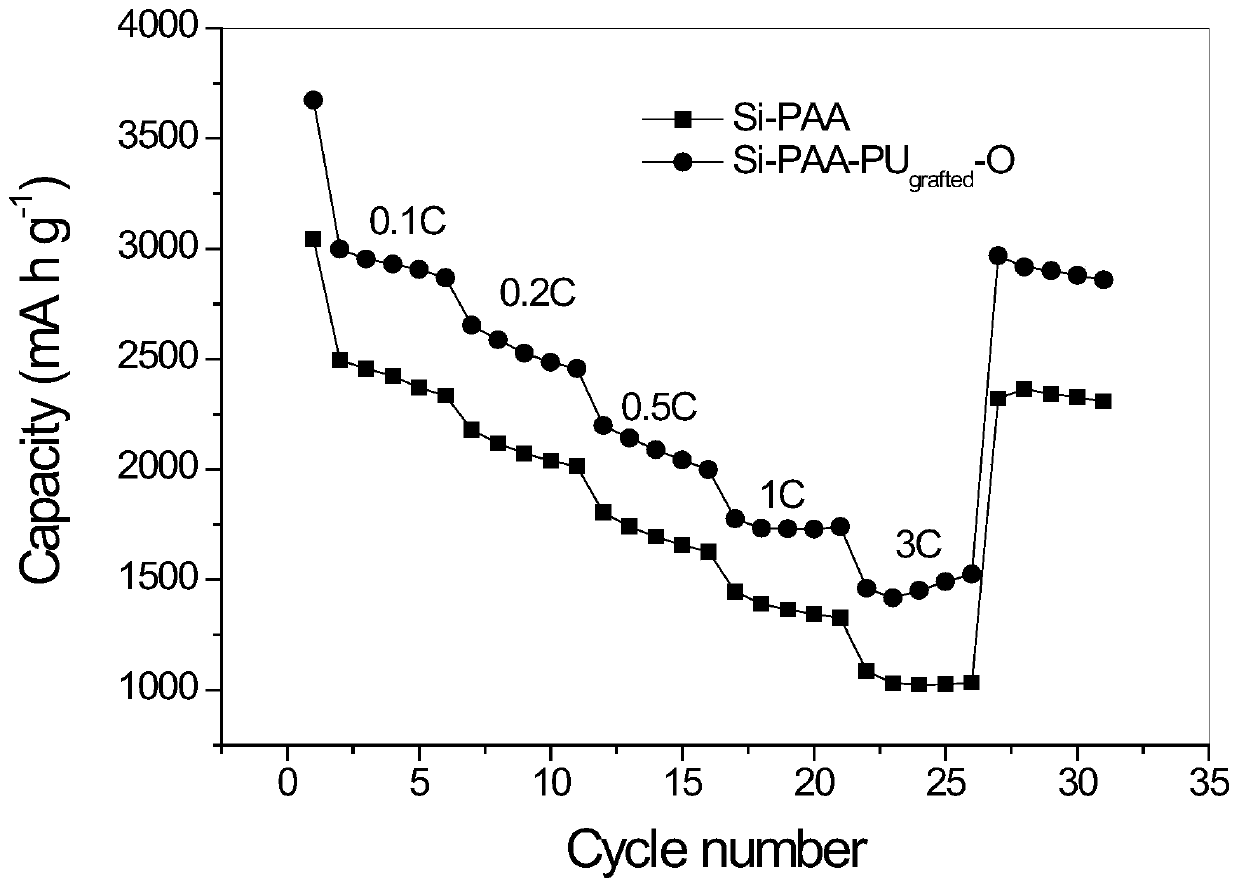
Binder with three-dimensional network structure and preparation method and application thereof
A technology of network structure and binder, which is applied in the direction of structural parts, electrical components, electrochemical generators, etc., can solve the problems of poor cycle performance of electrode materials, achieve improved cycle stability, improve the first charge and discharge efficiency, and improve Effect of the override characteristic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of organic solvent system polyacrylic acid / polyurethane three-dimensional network structure binder (structure Ⅰ):
[0043] (1) Preparation of isocyanate-terminated polyurethane prepolymer:
[0044] Add A4 molecular sieves into the Shrek reaction bottle, use acetylene flame to remove water, and at the same time evacuate the water and oxygen in the bottle; add N-methylpyrrolidone NMP after cooling to obtain anhydrous and oxygen-free NMP solvent. Polypropylene glycol PPG400 was dissolved in the above-mentioned NMP to obtain a 10% clear solution, and a certain amount of isophorone diisocyanate was added for polycondensation to obtain an isocyanate-terminated polyurethane prepolymer as component A.
[0045] (2) First, polyurethane (BASF TPU1195A10) and polyacrylic acid (Aladdin analytical grade, Mw 450,000) were dehydrated at 100° C. and 0.1 MP vacuum for 5 hours, respectively.
[0046] Dissolve 0.5g polyacrylic acid in anhydrous NMP, heat and stir at 60°C to ma...
Embodiment 2
[0052] Preparation of organic solvent system polyacrylic acid / polyurethane three-dimensional network structure binder (structure Ⅰ):
[0053] (1) Preparation of isocyanate-terminated polyurethane prepolymer:
[0054] Add A4 molecular sieve into the Shrek reaction bottle, use acetylene flame to remove water, and at the same time evacuate the water and oxygen in the bottle; after cooling, add N-methylpyrrolidone (NMP) to prepare anhydrous and oxygen-free NMP solvent. Dry the 500mL four-necked flask and the condensing reflux tube in advance. Polyethylene glycol was dehydrated in vacuum at 120°C and 0.1MPa for 5h. Under the protection of nitrogen, 22g of anhydrous NMP, 90g of vinyl acetate, and 88.14g of polyethylene glycol were added, condensed and refluxed at 80°C, and stirred for 1 hour until the solution was transparent and clear. Add 110.04 g of isophorone diisocyanate in one go, stir, and react for 3 hours. Dissolve 1,4-butanediol and hydroquinone in 15ml of ethyl acetate...
Embodiment 3
[0061] Preparation of organic solvent system polyacrylic acid / polyurethane grafted three-dimensional network structure binder (structure Ⅱ):
[0062] (1) Preparation of isocyanate-terminated low molecular weight polyurethane prepolymer (Mw 5,000)
[0063] Add A4 molecular sieves into the Shrek reaction bottle, use acetylene flame to remove water, and at the same time evacuate the water and oxygen in the bottle; add N-methylpyrrolidone NMP after cooling to obtain anhydrous and oxygen-free NMP solvent. Polytetrahydrofuran was dissolved in the above NMP to obtain a 0.5% clear solution, and 0.05 mol of isophorone diisocyanate was added to modify it into an isocyanate-terminated polyurethane prepolymer as component A.
[0064] (2) Preparation of isocyanate-terminated high molecular weight polyurethane (Mw 100,000)
[0065] Dry the 500mL four-necked flask and the condensing reflux tube in advance. Polytetrahydrofuran was vacuum dehydrated at 80°C and 0.1 MPa for 5 hours; under nit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com