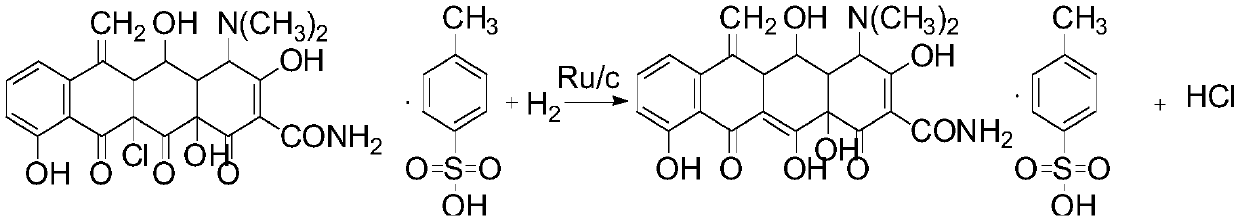
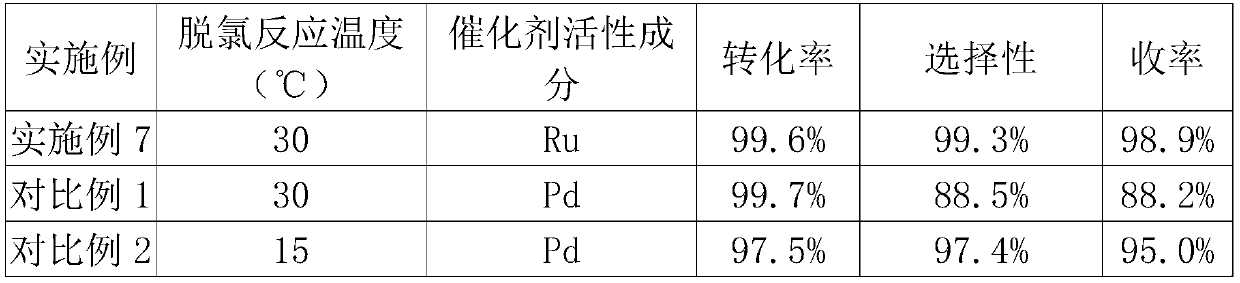
Preparation method of methacycline
A technology of oxytetracycline and oxytetracycline, which is applied in the field of preparation of oxytetracycline methacycline, can solve the problems of rising cost of catalytic hydrogenation, high cost of oxytetracycline methacycline, and difficult control of catalyst activity , to achieve good industrialization prospects, reduce production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
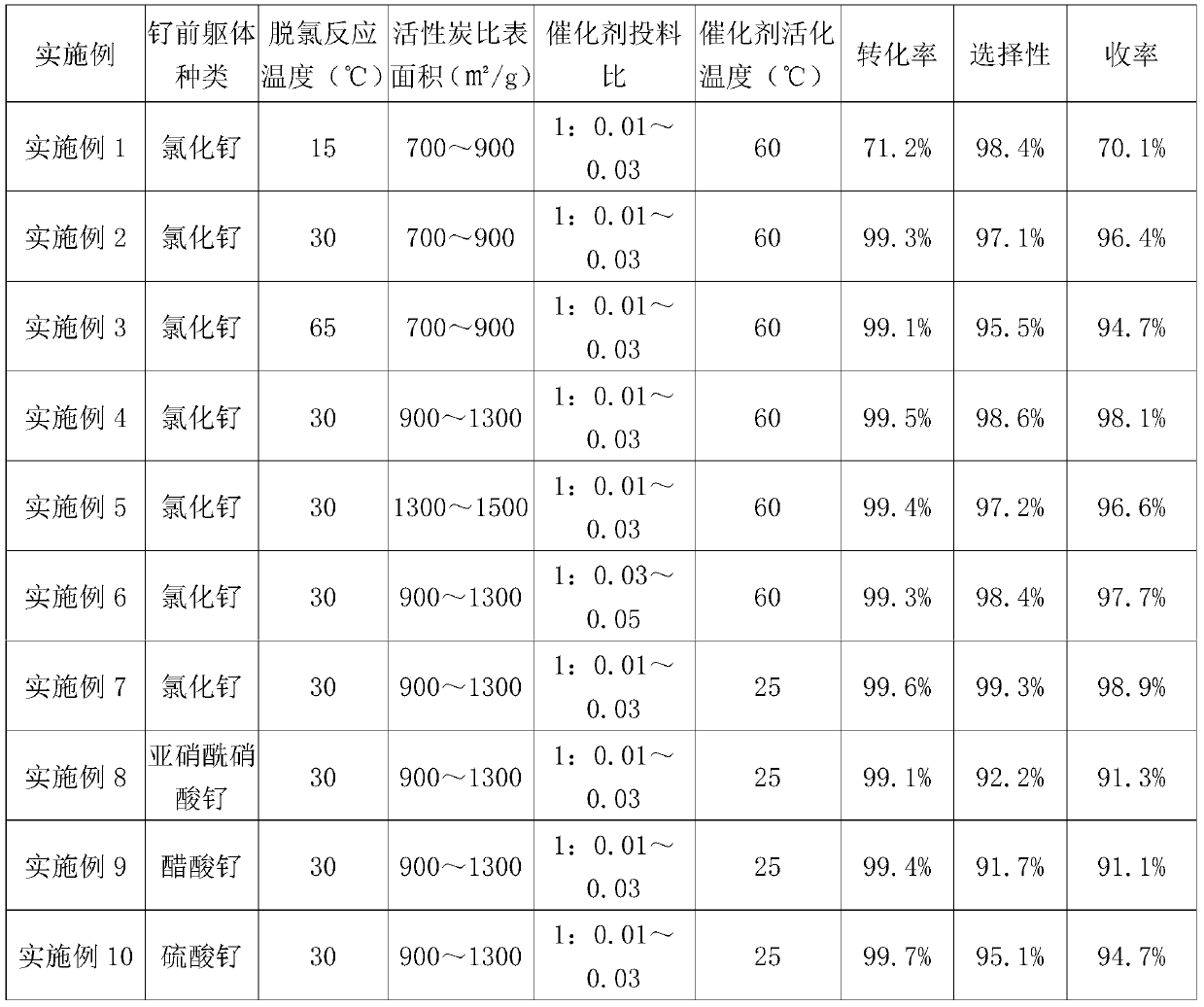
[0027] Add 300.34g of purified water into a 500ml reaction bottle equipped with a thermometer and mechanical stirring, add 30.02g of activated carbon with a specific surface area of 700-900㎡ / g, add dropwise 11.40ml of ruthenium trichloride aqueous solution with a concentration of 0.2g / ml, Heat and stir for 12 hours, put the impregnating solution into a 500ml autoclave, keep warm at 60°C, charge hydrogen and stir for 2.5 hours, and filter out 57.12g of wet catalyst.
[0028] Add 100.12g of solvent 50% ethanol water to a 500ml autoclave, drop into 20.03g of 11α-chloro-6methine oxytetracycline p-toluenesulfonate, keep warm at 15°C, drop into 0.78g of the ruthenium carbon catalyst prepared above, and charge hydrogen The reaction was stirred for 7 hours, and liquid phase analysis was carried out by sampling. The conversion rate was 71.2%, the selectivity was 98.4%, and the yield was 70.1%.
Embodiment 2
[0030] Drop into 50% ethanol water 100.23g as solvent in a 500ml autoclave, drop into 20.01g of 11α-chloro-6methine oxytetracycline p-toluenesulfonate, keep warm at 30°C, drop into the ruthenium carbon prepared in Example 1 Catalyst 0.74g, charged with hydrogen and stirred for 7 hours, sampling for liquid phase analysis, the conversion rate was 99.3%, the selectivity was 97.1%, and the yield was 96.4%.
Embodiment 3
[0032] In a 500ml autoclave, drop into solvent as 100.23g of 50% ethanol water, drop into 20.01g of 11α-chloro-6methine oxytetracycline p-toluenesulfonate, keep warm at 65°C, drop into the ruthenium carbon prepared in Example 1 Catalyst 0.75g, charged with hydrogen and stirred for 7 hours, liquid phase analysis by sampling, the conversion rate was 99.1%, the selectivity was 95.5%, and the yield was 94.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com