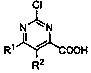
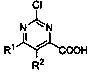
Continuous synthesis method of 2-chloropyrimidine-4-formic acid compound
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve the problems of good environmental protection and inability to take into account low cost, and achieve the effect of saving labor costs, taking into account costs, and reducing the risk factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
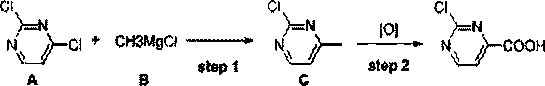
[0033] In formula I, R 1 and R 2 independently selected from hydrogen, alkoxy, aryl, benzyl or fluorine; the synthesis method comprises the following steps: S1, under the action of a non-noble metal catalyst, compound A and methyl Grignard reagent B are subjected to continuous methylation chemical reaction to obtain compound C; compound A is , compound C is , R 1 and R 2 With the same definition as above, the non-precious metal catalyst is one or more of iron salt, cobalt salt, nickel salt; S2, the compound C is subjected to continuous oxidation reaction under the action of oxygen to obtain 2-chloropyrimidine-4-carboxylic acids compound.
[0034]The present invention greatly improves the reaction efficiency and yield through the two-step continuous reaction, and the total yield of 2-chloropyrimidine-4-carboxylic acid compounds can also be increased to more than 70%, correspondingly greatly reducing the synthesis of the product cost. At the same time, the present inven...
Embodiment 1
[0052] Synthesized 2-chloropyrimidine-4-carboxylic acid in this embodiment, and concrete route is as follows:
[0053]
[0054] Step1 continuous methylation reaction:
[0055] 2,4-dichloropyrimidine 50g (compound A, 1.0equiv.), co-solvent N-methylpyrrolidone NMP 39.9g (1.2equiv.), catalyst FeCl 3 1.1 g (0.02 equiv.) was dissolved in 500 mL THF to obtain the first raw material solution. Then use the pump 1 to pump the first raw material solution into the coil of the coil reactor at a speed of 8.9g / min, and simultaneously use the pump 2 to pump 131g of methylmagnesium chloride Grignard reagent B into the coil with a speed of 2.2g / min In the tube, the coil is placed in an external bath at -55~-60°C for continuous methylation reaction. Wherein, the retention time of the first raw material liquid in the coil tube is 20min. The outlet of the coil is directly connected to a four-neck bottle with 200mL of ice water at 0~5°C. After feeding, the system is concentrated to remove T...
Embodiment 2
[0059] Compared with embodiment 1, be that the selected catalyst of step 1 is different
[0060] Step1 continuous methylation reaction:
[0061] 50g of 2,4-dichloropyrimidine (Compound A, 1.0equiv.), 39.9g (1.2equiv.) of N-methylpyrrolidone NMP as a cosolvent, and 0.93g (0.02equiv.) of catalyst NiCl2 were dissolved in 500mL THF to obtain the first raw material liquid. Then use the pump 1 to pump the first raw material solution into the coil of the coil reactor at a speed of 8.9g / min, and simultaneously use the pump 2 to pump 131g of methylmagnesium chloride Grignard reagent B into the coil with a speed of 2.2g / min In the tube, the coil is placed in an external bath at -55~-60°C for continuous methylation reaction. Wherein, the retention time of the first raw material liquid in the coil tube is 20min. The outlet of the coil is directly connected to a four-neck bottle with 200mL of ice water at 0~5°C. After the feeding is completed, the system is concentrated to remove THF, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com