Application of Pianzaihuang for preparing medicine capable of treating rheumatoid arthritis
A rheumatoid, Pien Tze Huang technology, applied in the field of medicine, can solve problems such as adverse drug reactions, MTX hypersensitivity, etc., and achieve the effect of good clinical application foundation and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Drug efficacy test of Pien Tze Huang in treating rheumatoid arthritis
[0023] 1. Animal modeling and administration:
[0024] Healthy DBA / 1J mice, male, SPF grade, 6-8 weeks old, for modeling: On day 0 (primary immunization), inject bovine type Ⅱ collagen and complete Freund's adjuvant emulsified intradermally at the base of the tail of the mice On the 21st day (boosting immunization), 100 μL / mouse of emulsifier consisting of bovine type II collagen and incomplete Freund's adjuvant was injected at the same site. The emulsifier used was bovine type II collagen with a concentration of 2 mg / mL was added dropwise to an equal volume of complete or incomplete Freund's adjuvant, and was emulsified with a homogenizer while dripping, with a rotation of 3 min and a stop of 1 min until the The emulsifier does not spread when it is dropped into the water, and the whole process is carried out in an ice bath (the emulsifier is ready for use).
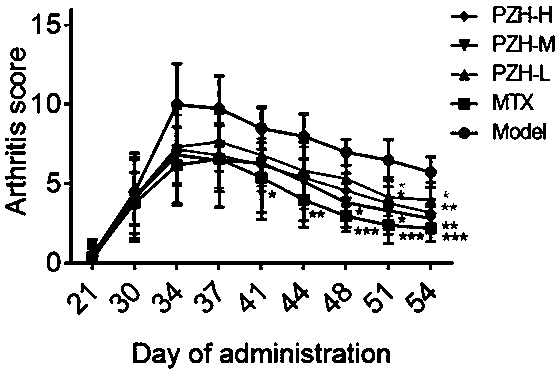
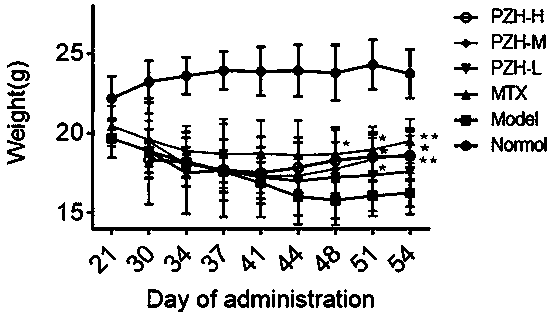
[0025] Grouping and admini...
Embodiment 2
[0049] Example 2 Toxicity study
[0050] Acute toxicity test (single administration): intragastric administration of Pien Tze Huang (12g / kg, 400 times the clinically intended dose) to SD rats for 3 times within 24 hours, and observe the toxicity produced within a certain period of time The results showed that: the rats in the test group had no obvious abnormal symptoms before and after three administrations; there was no abnormality in the general observation of the first 1-14 days after that, and no rats died at the end of the test; no pathological anatomy was observed with the naked eye. Obvious lesions and abnormalities, compared with the vehicle group, there was no statistical difference in body weight.
[0051] 180-day toxicity test: set up 270mg / kg, 900mg / kg, 3000mg / kg dose groups and vehicle control group, observe the toxic reaction and dose-response relationship of rats, whether there is delayed toxic reaction after drug withdrawal and the reversibility of toxic reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com