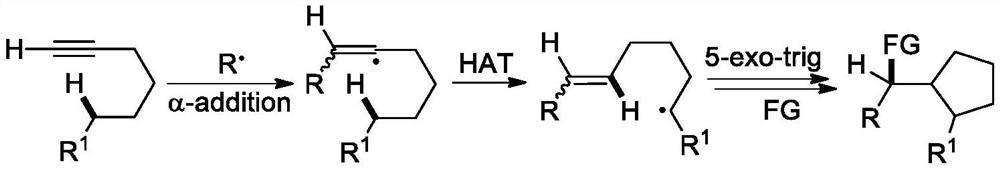
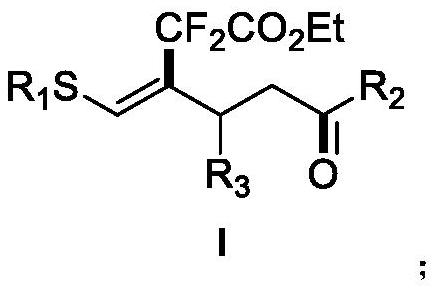
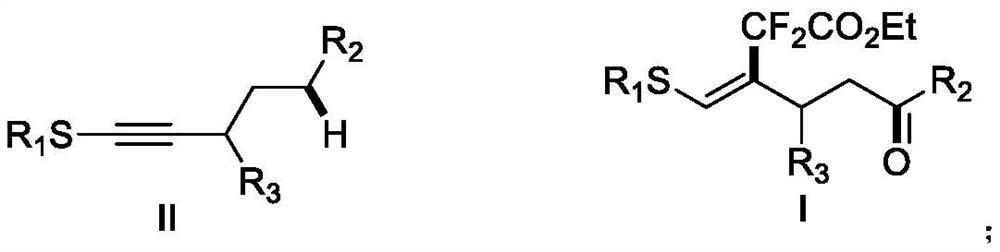A kind of (z)-4-difluoroalkyl-5-sulfanyl-4-pentenone derivative and its preparation method
A technology of difluoroalkyl and sulfanyl, applied in the field of -4-difluoroalkyl-5-sulfanyl-4-pentenone derivatives and its preparation, can solve the problem of not obtaining olefin addition products and other problems, to achieve the effect of a wide range of substrates, good application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take a dry reaction tube, add hi-tris(2-phenylpyridine)iridium (2.6mg, 0.004mmol), silver carbonate (110.3mg, 0.4mmol), ethyl bromodifluoroacetate under nitrogen atmosphere (81.2 mg, 0.40 mmol), alkyne sulfide represented by structural formula 1a (38 mg, 0.2 mmol) were then added to 2 mL of dry dimethyl sulfoxide to form a reaction system. The system was stirred under a 15W blue light lamp at room temperature 25°C for 12h, quenched by adding 15mL of water, extracted three times with ethyl acetate (10mL), combined, the organic phase was washed with saturated edible water, and dried over anhydrous sodium sulfate. After the organic phase was concentrated, it was separated by silica gel (300-400 mesh) column chromatography to obtain 50 mg of a light yellow liquid represented by structural formula 2a, with a yield of 78%.
[0028] Product Spectrum Analysis 1 H NMR (600MHz, CDCl 3 )δ7.98–7.97(m,2H),7.59(t,J=7.4Hz,1H),7.48(t,J=7.7Hz,2H),6.44(s,1H),4.33(q,J=7.1 Hz, 2H), 3.21...
Embodiment 2
[0032] Except that the alkyne thioether derivative shown in structural formula 1b was used instead of the alkyne thioether derivative shown in structural formula 1a in Example 1, the remaining operating steps were the same as in Example 1, yield: 67%, light yellow liquid shown in structural formula 2b.
[0033] Product Spectrum Analysis 1 H NMR (600MHz, CDCl 3 )δ7.96(d, J=7.3Hz, 2H), 7.57(t, J=7.4Hz, 1H), 7.47(t, J=7.7Hz, 2H), 6.47(s, 1H), 4.30(q, J=7.1Hz, 2H), 3.20(t, J=7.3Hz, 2H), 2.73–2.67(m, 4H), 1.30(t, J=7.1Hz, 3H), 1.27(t, J=7.4Hz, 3H); 13 C NMR (151MHz, CDCl 3 )δ198.8, 163.4(t, J=35.4Hz), 136.7, 133.4(t, J=6.0Hz), 133.1, 128.6, 128.1(t, J=23.4Hz), 127.9, 113.9(t, J=251.6Hz) ,63.0,37.9,29.2,27.6(t,J=3.6Hz),15.3,13.8; 19 F NMR (565MHz, CDCl 3 )δ-100.4; HRMS (ESI) calcd for C 17 h 20 f 2 o 3SNa(M+Na) + 365.0993, found 365.1002.
[0034] The reaction formula is as follows:
[0035]
Embodiment 3
[0037] Except that the alkyne thioether derivative shown in structural formula 1c was used to replace the alkyne thioether derivative shown in structural formula 1a in Example 1, the rest of the operation steps were the same as in Example 1, yield: 83%, light yellow liquid shown in structural formula 2c.
[0038] Product Spectrum Analysis 1 H NMR (600MHz, CDCl 3 )δ7.86(d, J=8.2Hz, 2H), 7.26(d, J=7.8Hz, 2H), 6.41(s, 1H), 4.32(q, J=7.1Hz, 2H), 3.16(t, J=7.3Hz, 2H), 2.68(t, J=7.4Hz, 2H), 2.41(s, 3H), 2.29(s, 3H), 1.32(t, J=7.1Hz, 3H); 13 C NMR (151MHz, CDCl 3 )δ198.4, 163.4(t, J=35.0Hz), 143.9, 135.0(t, J=5.8Hz), 134.3, 129.3, 128.1, 127.6(t, J=23.4Hz), 114.0(t, J=252.0Hz) ,63.0,37.8,27.6(t,J=3.6Hz),21.6,18.4,13.9; 19 F NMR (565MHz, CDCl 3 )δ-100.7; HRMS (ESI) calcd for C 17 h 20 f 2 o 3 SNa(M+Na) + 365.0993, found 365.0995.
[0039] The reaction formula is as follows:
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com