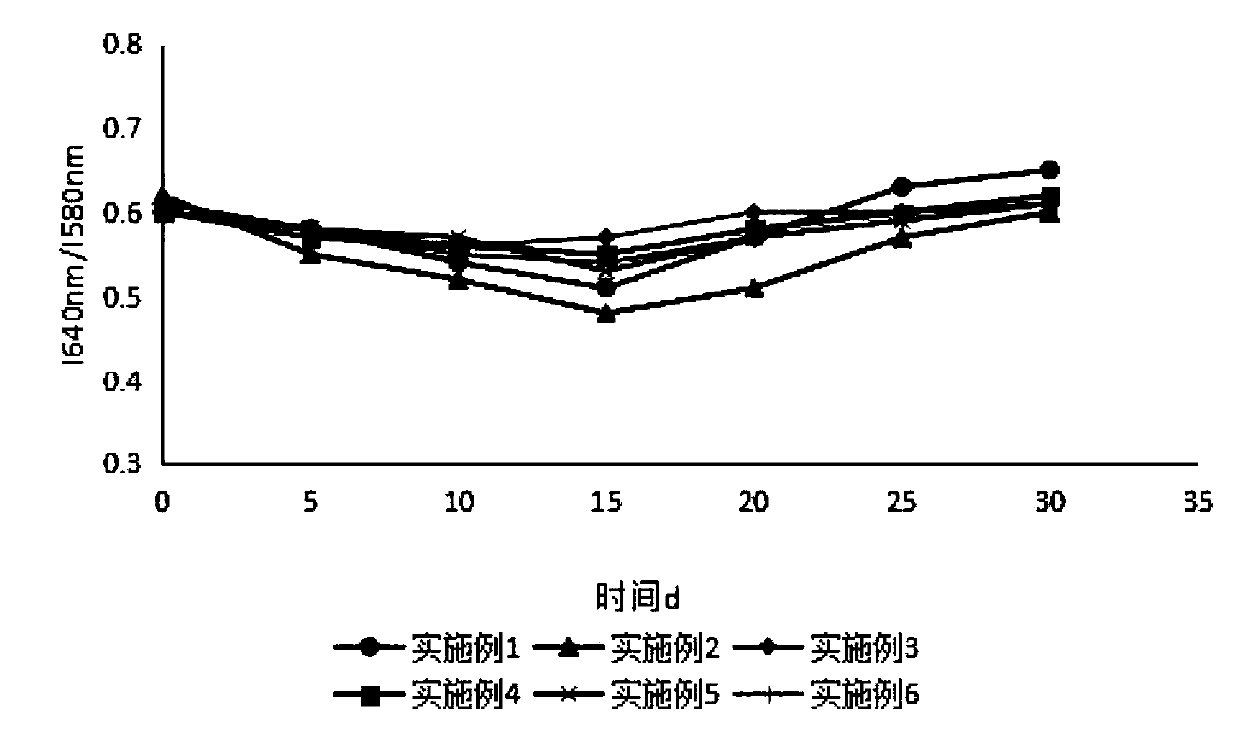
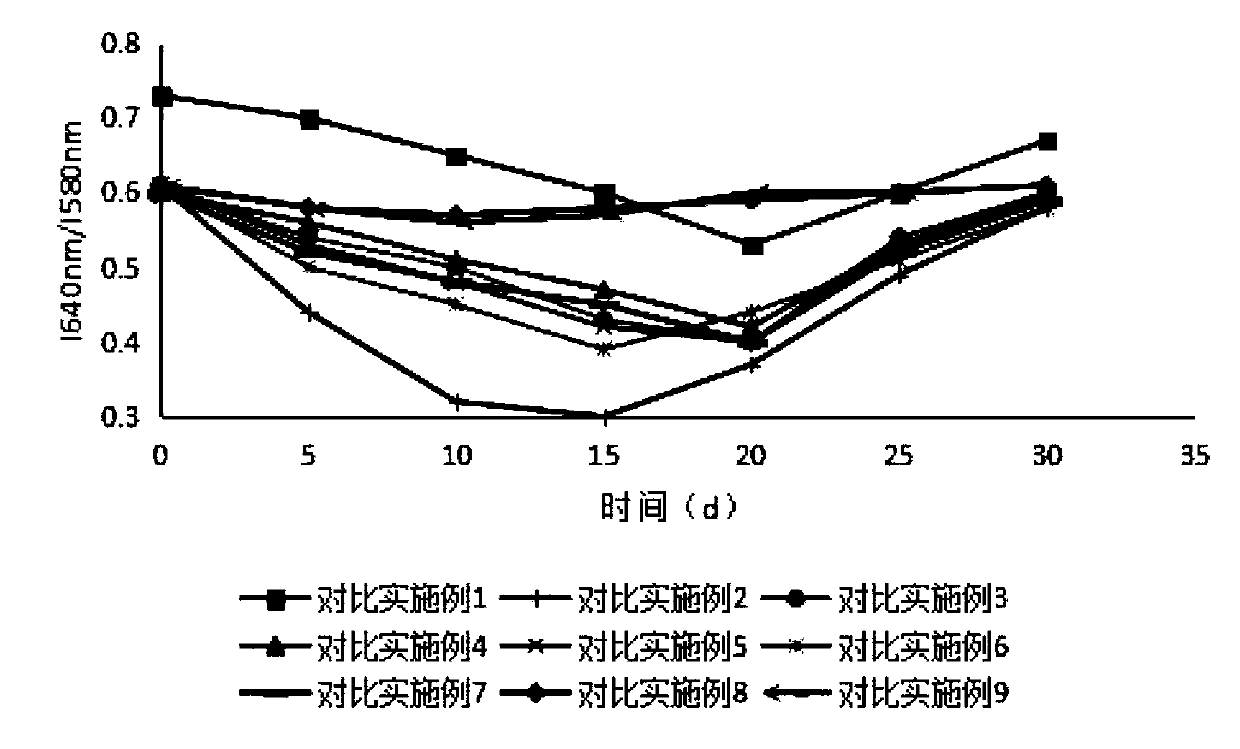
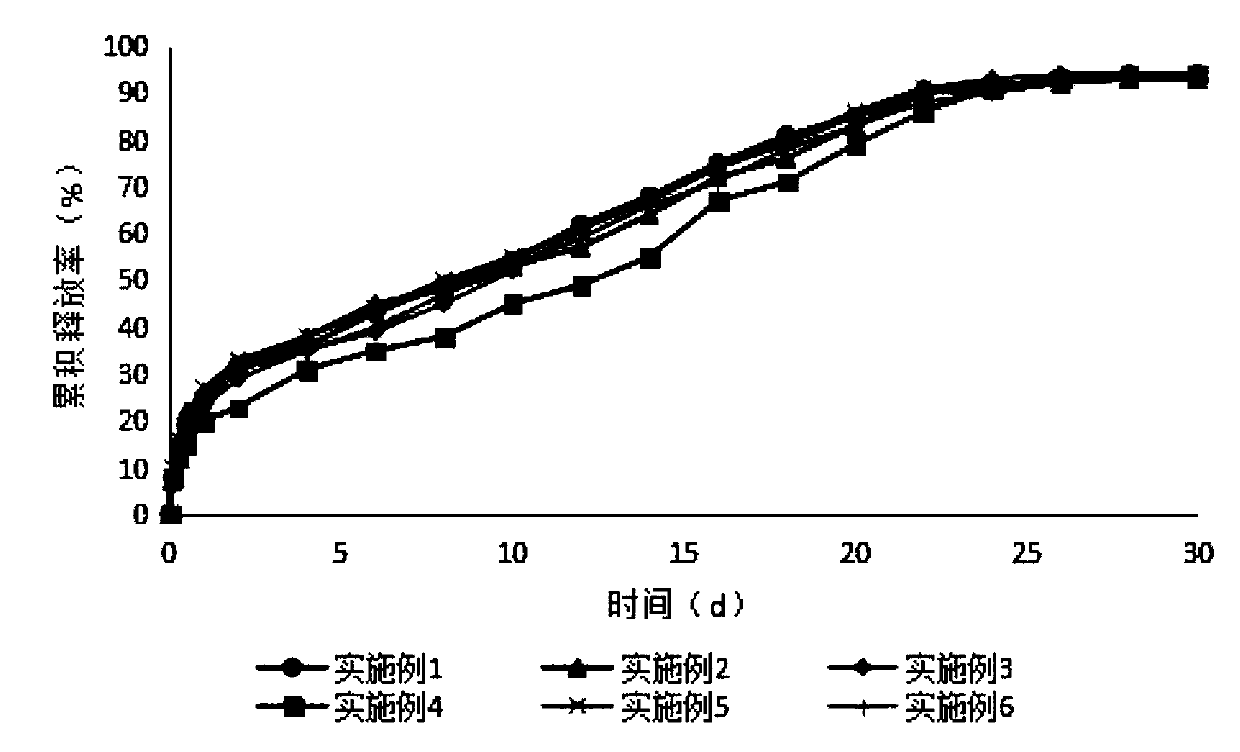
Sustained-release microsphere preparation of glucagon-like peptide-1 analogue and preparation method of microsphere preparation
A technology of sustained-release microsphere preparations and glucagon, which is applied in the direction of non-active ingredients of polymer compounds, pharmaceutical formulations, microcapsules, etc., and can solve the instability, encapsulation efficiency and loading of GLP-1 analog sustained-release preparations. Unsatisfactory drug dosage and unable to solve the problems of peptide instability, etc., to achieve good drug release characteristics, improve drug safety, and improve drug compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Prescription
[0042]
[0043] 2) Preparation process:
[0044] a) Liraglutide and hydroxypropyl-β-cyclodextrin are dissolved in sterile water for injection as the inner water phase, and the mass-volume concentration of liraglutide in the inner water phase is 200 mg / mL;
[0045] b) The polymer blend and the release modifier are added to a mixed solution of dichloromethane and ethyl acetate (1:1, v / v) to dissolve, as the organic phase, the mass-volume of the polymer blend in the organic phase The concentration is 300mg / ml;
[0046] c) adding the internal aqueous phase of step a) to the organic phase of step b) under high-speed stirring at 23000 rpm, emulsifying for 30 seconds, and mixing evenly to obtain colostrum;
[0047] d) Add the colostrum obtained in step c) dropwise into the polyvinyl alcohol solution at 4°C under the stirring condition of 1500rpm, stir at a low speed of 600rpm to evaporate the organic solvent, and collect the microspheres through a 1200-m...
Embodiment 2
[0050] 1) Prescription
[0051]
[0052] 2) Preparation process: the preparation process is the same as in Example 1.
Embodiment 3
[0054] 1) Prescription
[0055]
[0056]
[0057] 2) Preparation process: the preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com