Method for preparing sustained-release tablets through granulating by adopting bonding agents in atomizing states
A technology of adhesives and sustained-release tablets, which is applied in the field of preparation of pharmaceutical preparations, can solve problems such as low yield of finished products, fluctuations in the release rate of finished sustained-release tablet preparations, unfavorable drug safety for patients taking drugs, etc., to reduce release rate fluctuations, The effect of reducing drug safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A method for preparing sustained-release tablets granulated in an atomized state of an adhesive, comprising the following steps:
[0019] (1) Weigh 20% of gliclazide, 35% of hypromellose, 34% of microcrystalline cellulose, and 10% of lactose by weight percentage and mix to obtain mixture 1;
[0020] (2) Spray water that accounts for 7% of the weight of the mixture 1 into the mixture 1 in a 120-degree solid conical atomization state, wet granulate, pass 16 mesh granulation, and dry the granules to a water content of 1%-7%. 16-mesh granules; mixed with magnesium stearate accounting for 1.0% of the weight of the whole granules, compressed into sustained-release tablets, and then film-coated to obtain the finished product of sustained-release tablets.
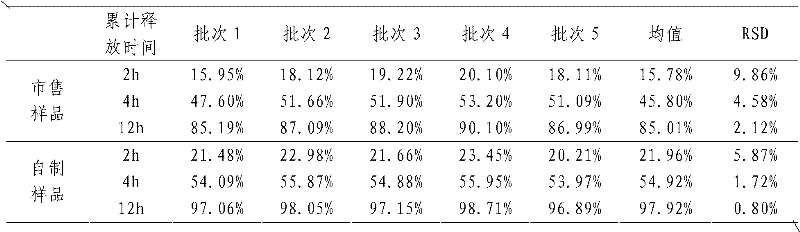
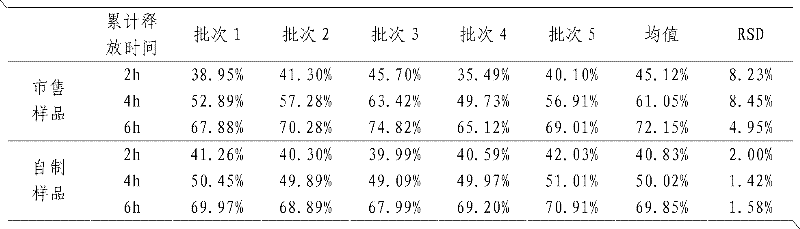
[0021] In this example, the average finished product yield of the prepared sustained-release tablets is 99.35%, while the finished product yield prepared by the traditional sustained-release tablet preparation method is only...
Embodiment 2
[0027] A method for preparing sustained-release tablets granulated in an atomized state of an adhesive, comprising the following steps:
[0028] (1) Weigh 10% nifedipine, 35% hypromellose, and 54% lactose by weight percentage and mix to obtain mixture 1;
[0029] (2) Water 5% by weight of the mixture 1 is sprayed into the mixture 1 in a 65-degree hollow square atomized state for wet granulation, passed through 16 mesh granulation, dried to a water content of 1%-7%, passed through 16 Then mix with magnesium stearate accounting for 1.0% of the weight of the whole granules, compress into sustained-release tablets, and then film-coat to obtain the finished product of sustained-release tablets.
[0030] In this example, the average finished product yield of the prepared sustained-release tablets was 98.72%, while the finished product yield of the traditional sustained-release tablet preparation method was only 87.21%. The finished nifedipine sustained-release tablets prepared by t...
Embodiment 3
[0036] A method for preparing sustained-release tablets granulated in an atomized state of an adhesive, comprising the following steps:
[0037] (1) Weigh 20% ibuprofen, 20% hypromellose, 34% precrossified starch, and 25% lactose by weight percentage and mix to obtain mixture 1;
[0038] (2) Spray water accounting for 10% of the weight of mixture 1 into mixture 1 in a 90-degree fan-shaped atomization state, wet granulate, pass through 16 mesh for granulation, dry the granules to a water content of 1%-7%, pass through 16 mesh Whole granules; mixed with micropowder silica gel accounting for 1.0% of the weight of the whole granules, compressed into sustained-release tablets, and then film-coated to obtain finished sustained-release tablets.
[0039] In this example, the average finished product yield of the prepared sustained-release tablets is 98.95%, while the finished product yield of the traditional sustained-release tablet preparation method is only 88.75%. The ibuprofen su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com