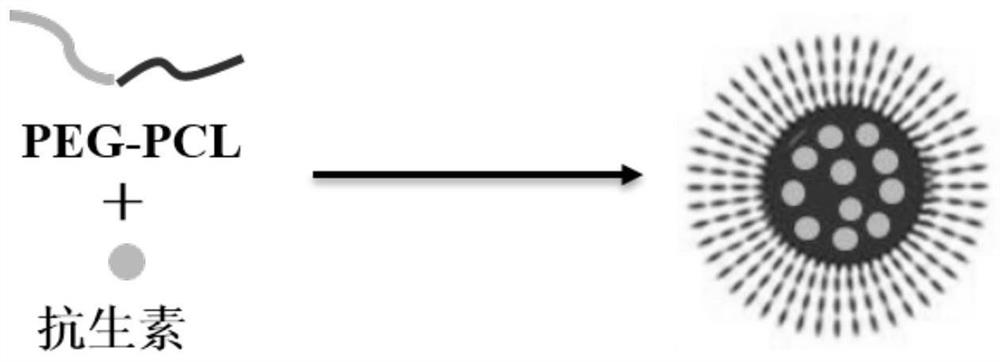
A kind of polycaprolactone-polyethylene glycol nanomicelle loaded with hydrophobic antibiotic and its preparation and application
A technology of nanomicelle and polycaprolactone, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and antibacterial drugs, can solve the problem that the delivery system is unstable and cannot play a targeting role. and other problems to achieve the effect of improving in vivo stability, increasing drug intake, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 carries the preparation method of erythromycin micelles
[0049] The drug loading is the percentage of the mass of erythromycin to the mass of the polymer carrier material.
[0050] The polymer carrier material PCL used in this embodiment 2000 - MPEG 2000 and PCL 5000 -PEG 2000 The molecular weights are: PEG and MPEG are both 2000Da, and PCL is 2000Da and 5000Da.
[0051] For a schematic diagram of the synthesis construction, see figure 1 .
[0052] The preparation method of blank micelles, comprises the steps:
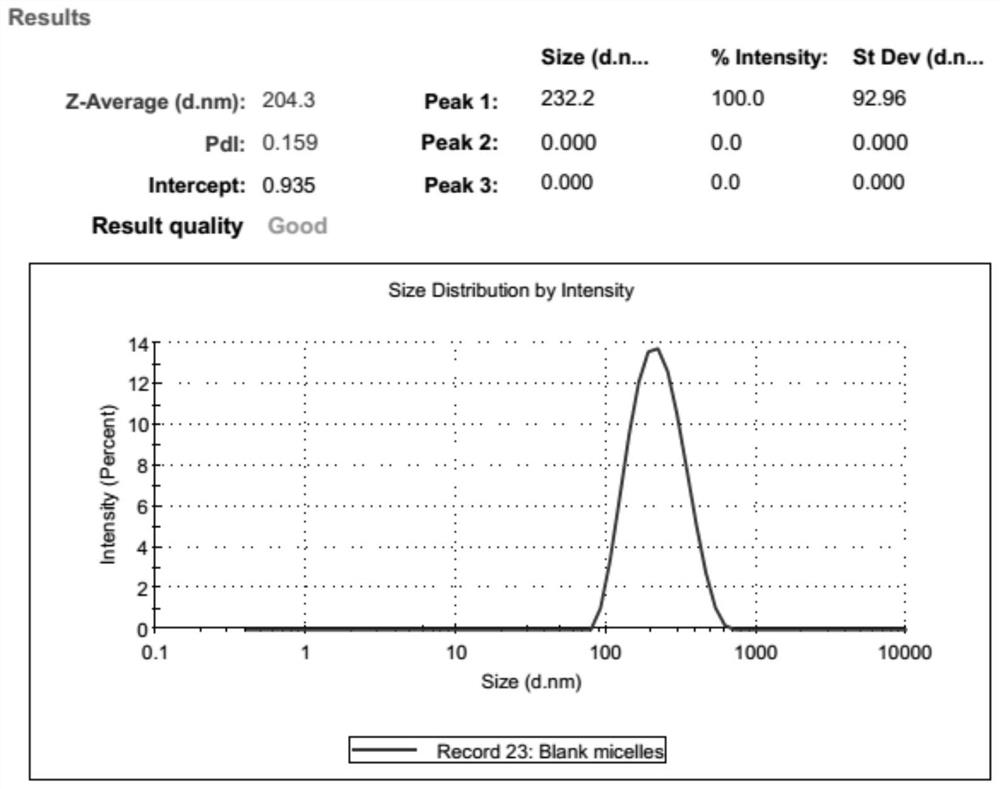
[0053] the PCL 2000 - MPEG 2000 and PCL 5000 -PEG 2000 Mix according to the mass ratio of 0:1, 0.1:1, 0.3:1, 0.5:1, 0.8:1, 1:1, 1:0, co-dissolve in 10mL tetrahydrofuran and shake until completely dissolved and mix well; then follow the method of tetrahydrofuran and Deionized water volume ratio (3-9): 1 Add deionized water drop by drop. Remove tetrahydrofuran by rotary evaporation in a round-bottomed flask at 30-50°C under vacuum to obta...
Embodiment 2
[0059] The preparation method of embodiment 2 loaded erythromycin micelles
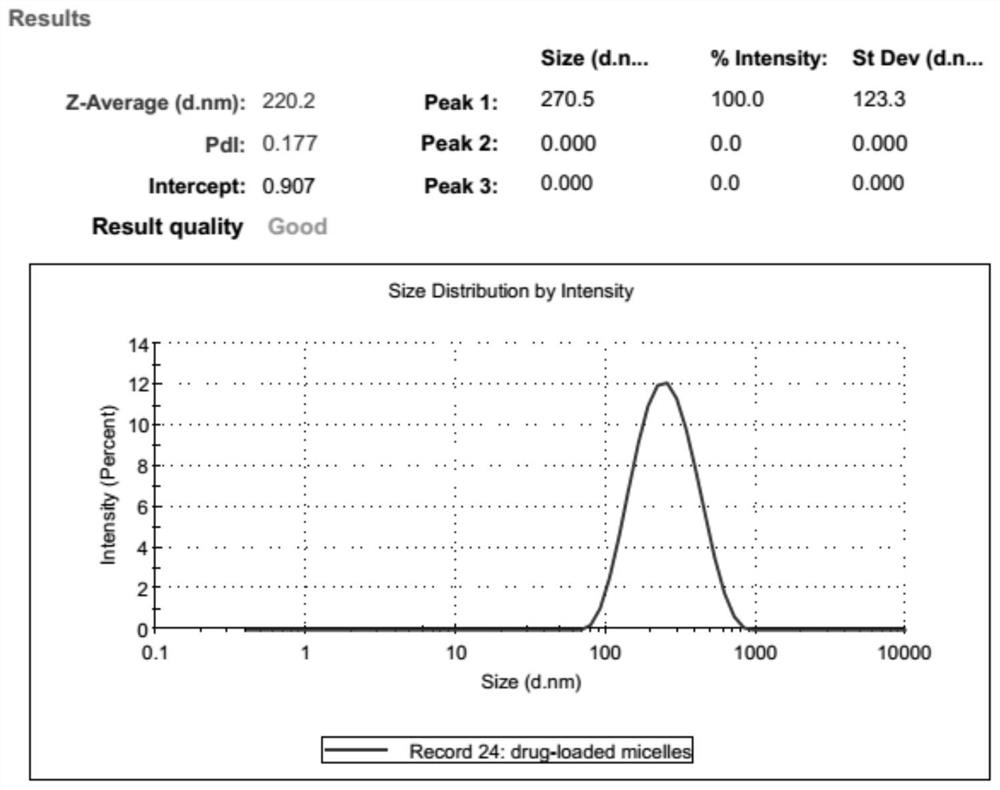
[0060] The preparation method of loading erythromycin micelles, comprises the steps: the PCL 2000 - MPEG 2000 and PCL 5000 -PEG 2000 Mix according to the mass ratio of 0.5:1, then add 1%, 5%, 10%, 15%, 20%, 25% of the total mass of the polymer carrier material erythromycin, dissolve in 2mL chloroform and shake until completely dissolved and Mix well; then add deionized water dropwise according to the volume ratio of deionized water to chloroform 1: (5-10). Remove chloroform by rotary evaporation in a round bottom flask at 30-50°C under vacuum to obtain a pan-opalescent micellar solution, and then sonicate in a water bath at 65°C for 10 minutes to further form a uniform micellar system. After filtering through a 0.22 μm microporous membrane to remove unencapsulated drug (erythromycin), it can be stored at 4°C for subsequent experiments.
[0061] The particle size and polydispersity coefficient of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com