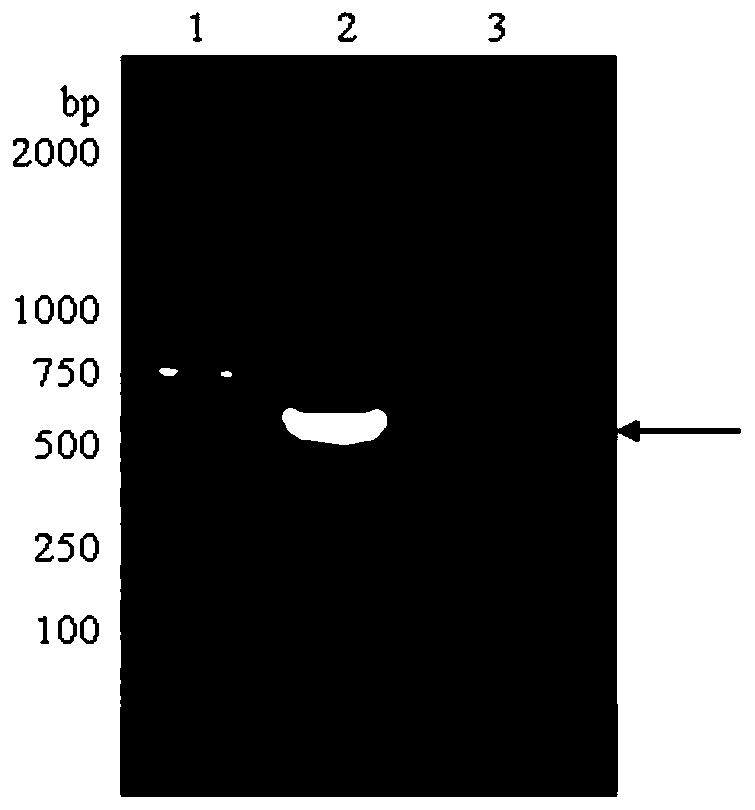
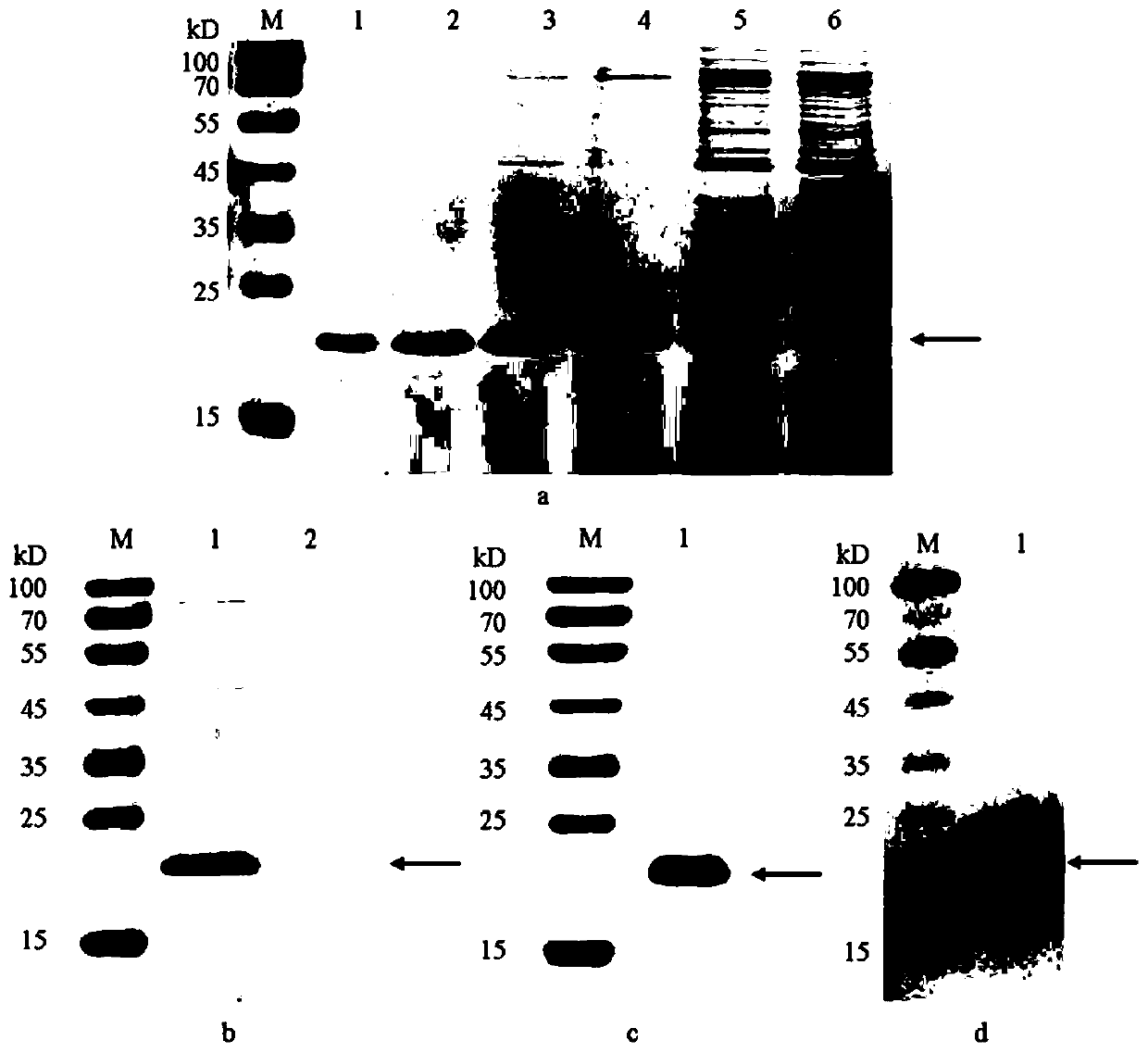
Method of obtaining human recombinant fibronectin by utilizing genetic engineering
A technology of fibronectin and protease, applied in the field of genetic engineering, can solve the problems of low biological activity and low amount of artificially extracted fibronectin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0037] 1. Artificially synthesized a recombinant human fibronectin fusion protein, and the fibronectin functional fragment in the recombinant fibronectin has a full length of 180 amino acids. The nucleotide sequence of recombinant human fibronectin after codon optimization is:
[0038] SEQ ID NO:2
[0039] 5’-ATGCACCACCACCACCACCACAATAGTGATTCCGAGTGCCCTCTTAGTCACGATGGCTATTGCCTTCACGATGGCGTCTGCATGTACATCGAAGCTTTGGACAAGTACGCTTGTAACTGTGTTGTTGGTTACATCGGTGAAAGATGTCAATACAGAGACTTGAAGTGGTGGGAATTGAGAGGTTCTGAAGGTTCTGAAGGTGAAGGTGGTTCTGAAGGTTCTGAAGGTGAAGGTGGCAGCGAAGGCAGCGAAGGCGAAGGCGGCAGCGAAGGCAGCGAAGGCGAAGGCGGTTCTGAAGGTTCTGAAGGTGAAGGTGGTTCTGAAGGTTCTGAAGGTGAAGGTATTACTTACGGTGAAACTGGTGGTAACTCTCCAGTTCAAGAATTTACTGTTCCAGGTTCTAAGTCTACTGCTACTATTTCTGGTTTGAAGCCAGGTGTTGATTACACTATTACTGTTTACGCTGTTACTGGTAGAGGTGACAGTCCTGCCAGTAGTAAACCTATCTCCATCAACTATAGGACCGAGATCGACAAACCTTAA-3’或
[0040] SEQ ID NO:3
[0041] 5’-ATGCACCACCACCACCACCACAATAGTGATTCCGAGTGCCCTCTTAGTCACGATGGCTATTGCCTTCACGATGGCGTCTGCATGTACATCGAAGCTTT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com