Palladium-charcoal catalyst as well as preparation method and application thereof
A palladium-carbon catalyst and a catalyst technology, which are applied in the directions of catalyst activation/preparation, hydrogenation preparation, chemical instruments and methods, etc., can solve the problems of low utilization rate of palladium atoms, loss of palladium metal, poor catalyst stability, etc., and avoid the recovery of palladium metal. Problems, blocking migration and shedding, effects of high conversion and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The invention provides a preparation method of palladium carbon catalyst. The preparation method comprises the following steps:
[0045] S1, pretreating the carrier;
[0046] S2. Immersing the carrier in the palladium metal precursor solution, washing and drying to obtain the catalyst precursor;
[0047] S3. Carrying out electrochemical in-situ reduction on the catalyst precursor, so that the palladium ions are reduced to metal palladium to prepare a palladium-carbon catalyst.
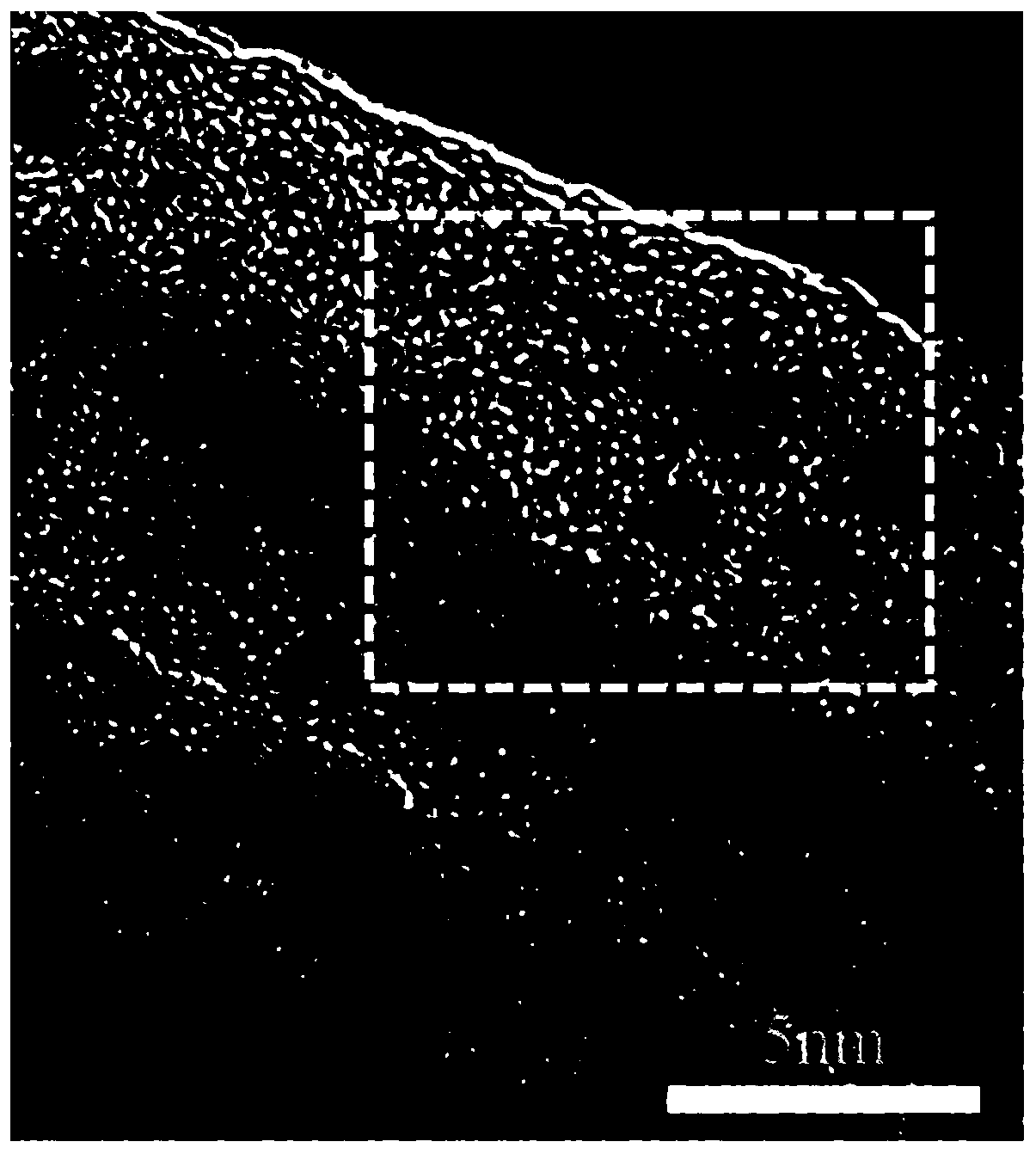
[0048] In the above step S1, the carrier in the pretreatment of the carrier includes at least one of activated carbon, carbon nanotubes, carbon fibers, and nitrogen-doped hierarchical porous carbon. Compared with ordinary carbon materials, nitrogen-doped carbon materials have Some unique advantages, such as nitrogen doping can change the local electronic structure of carbon materials, facilitate the dispersion of noble metal nanoparticles, and improve the activity and stability of catalysts th...
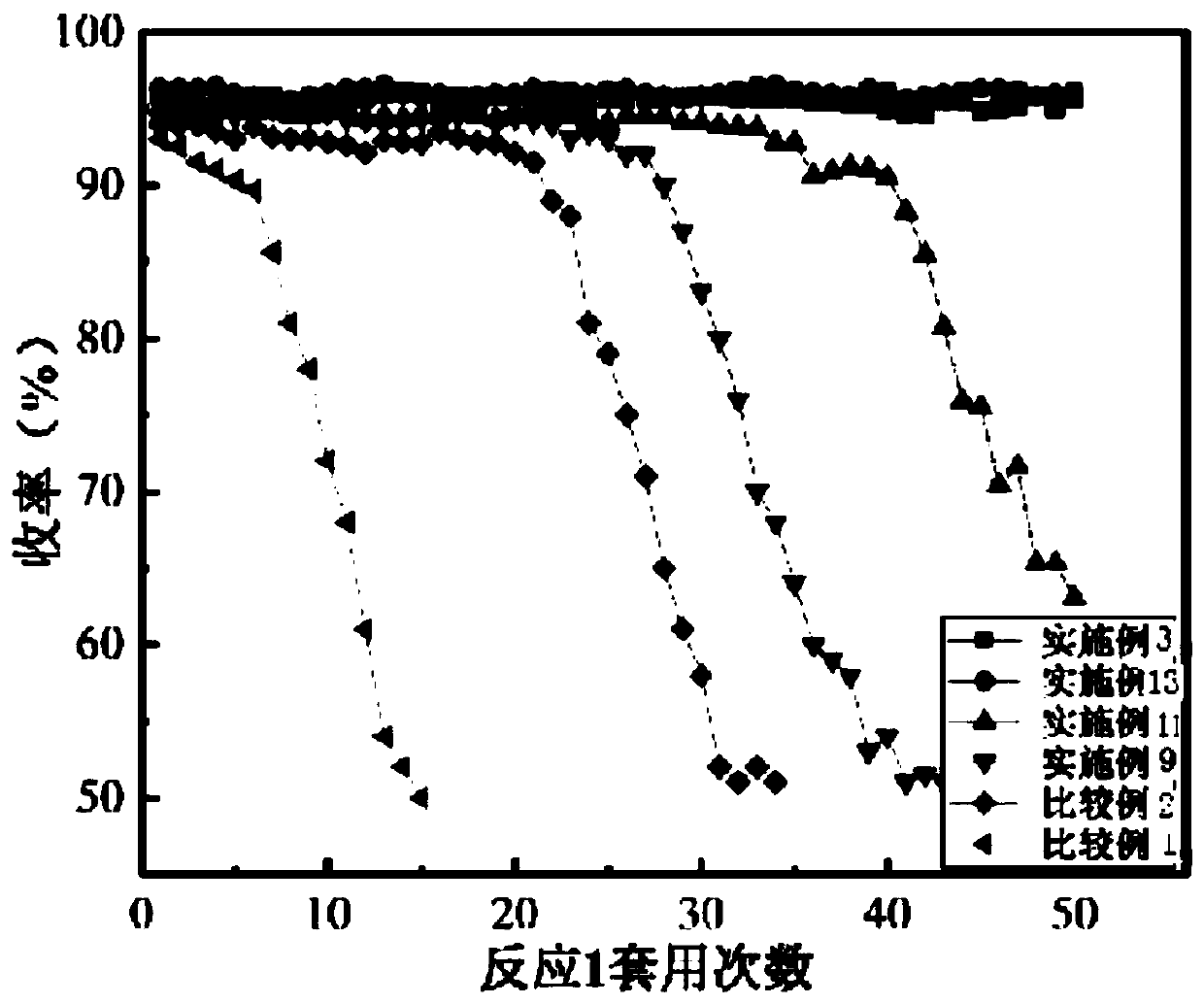
Embodiment 1
[0109] Weigh 1g of carbon nanotubes, first acid-treat the carbon nanotubes, put the carbon nanotubes into 50mL of 1mol / L H 2 SO 4 solution, and heated to reflux at 80° C. for 8 hours, and filtered to obtain a black powder after cooling down to room temperature. The black powder was washed with deionized water several times until the aqueous solution became neutral. Then the carbon nanotubes are heat-treated, the black powder is placed in an atmosphere of high-purity nitrogen, and calcined at a temperature of 1000° C. for 2 hours.
[0110] Weigh 0.5g of treated carbon nanotubes into 20mL of deionized water, sonicate for 1 hour, measure and add 0.05mL of PdCl 2 Solution (palladium concentration is 0.05g / mL), ultrasonication for 1 hour; centrifugation, remove the solution, add 20mL deionized water to wash, centrifuge, repeat 5 times, collect black powder, vacuum freeze-dry for 12 hours to obtain the catalyst precursor. Under the three-electrode system with the electrolyte of 1m...
Embodiment 2
[0112] This embodiment is basically the same as embodiment 1, the only difference is: measure 0.1mL of PdCl 2 Solution (palladium concentration is 0.05g / mL), prepares palladium carbon catalyst B, and its palladium loading is 1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com