Antigen and adjuvant co-delivery nanometer vaccine applied to liver cancer immunization therapy
An immunotherapy and nano-vaccine technology, applied in the field of biomedicine, can solve the problems of high cost of tumor vaccine raw materials, unfavorable large-scale processing and production, low immunotherapy effect, etc., and achieves easy repeatability of the preparation method, good application prospects, and enhanced immunotherapy. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of antigen and adjuvant co-delivery nano-vaccine applied to the immunotherapy of liver cancer comprises the following steps:
[0035] Step 1, the GPC3 127-136 Aqueous solution and polyethylenimine aqueous solution are mixed to obtain PC3 127-136 Mix solution with polyethyleneimine, then mix sodium alginate aqueous solution and CpG aqueous solution to obtain sodium alginate and CpG mixed solution, wherein GPC3 127-136 The concentration of aqueous solution is 0.5 mg / ml~4 mg / ml; the concentration of polyethyleneimine aqueous solution is 0.5 mg / ml~3 mg / ml; the concentration of CpG aqueous solution is 0.5 mg / ml~4 mg / ml; sodium alginate The concentration of the aqueous solution is 0.5 mg / ml~2 mg / ml; GPC3 127-136 The mass ratio between the aqueous solution and the polyethyleneimine aqueous solution is 1:0.05-0.5; the mass ratio between the sodium alginate aqueous solution and the CpG aqueous solution is 1:0.8-1.5.
[0036] Step 2: Add the mixed soluti...
Embodiment 1
[0038] Preparation of Nanovaccine:
[0039] GPC3 127-136Aqueous solution (1mg / ml, 0.5ml) was mixed with PEI aqueous solution (0.5mg / ml, 0.2ml), then ALG aqueous solution (0.5mg / ml, 0.5ml) was mixed with CpG aqueous solution (0.5mg / ml, 0.5ml), Under the condition of stirring, add the mixed solution of ALG and CpG to GPC3 dropwise 127-136 and PEI mixed solution, stirred for 5 minutes to obtain antigen and adjuvant co-delivery nano-vaccine.
[0040] Such as figure 1 As shown, the particle size distribution of the nano-vaccine obtained in this embodiment is relatively uniform, and the average particle size is 111.9nm.
[0041] Evaluation of nano-vaccine promoting DC maturation:
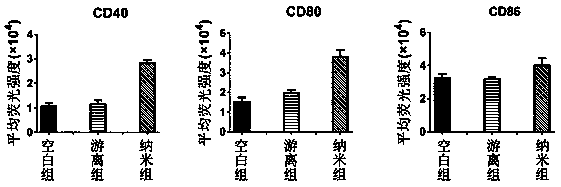
[0042] Collect the DC of immature on the sixth day, press 10 6 cells per well, spread DC on 24-well plate, and add nano-vaccine, GPC3 after 2 hours of attachment 127-136 The concentration was 10 micrograms per milliliter, and the culture was continued for 48 hours. The cells were collected in a cen...
Embodiment 2
[0045] Preparation of Nanovaccine:
[0046] GPC3 127-136 Mix aqueous solution (2mg / ml, 1ml) and PEI aqueous solution (2mg / ml, 0.3ml), then mix ALG aqueous solution (2mg / ml, 0.1ml) and CpG aqueous solution (2mg / ml, 0.1ml), under stirring conditions, Add ALG and CpG mixed solution dropwise to GPC3 127-136 and PEI mixed solution, stirred for 2 minutes to obtain antigen and adjuvant co-delivery nano-vaccine.
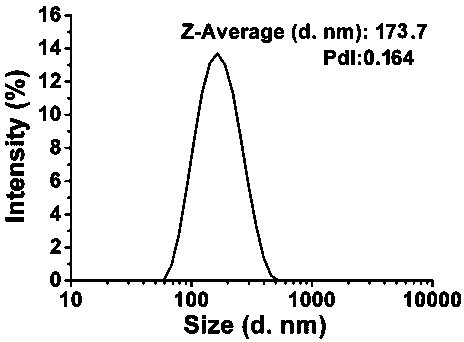
[0047] Such as image 3 As shown, the particle size distribution of the nano-vaccine obtained in this example is relatively uniform, with an average particle size of 173.7nm.
[0048] Evaluation of nano-vaccine promoting DC cytokine secretion:
[0049] Collect the DC of immature on the sixth day, press 10 6 cells per well, spread DC on 24-well plate, and add nano-vaccine, GPC3 after 2 hours of attachment 127-136 The concentration was 10 micrograms per milliliter, and the culture was continued for 48 hours. The cells were collected in a centrifuge tube by gentle blowin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com