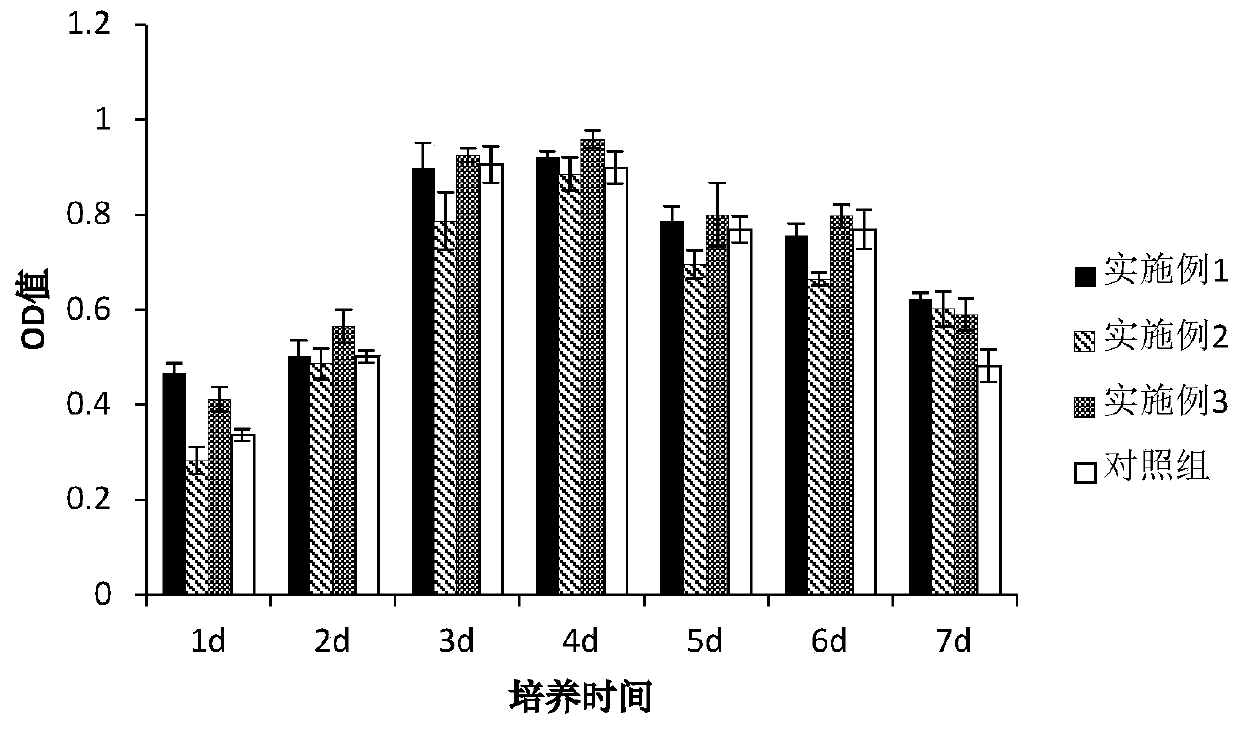
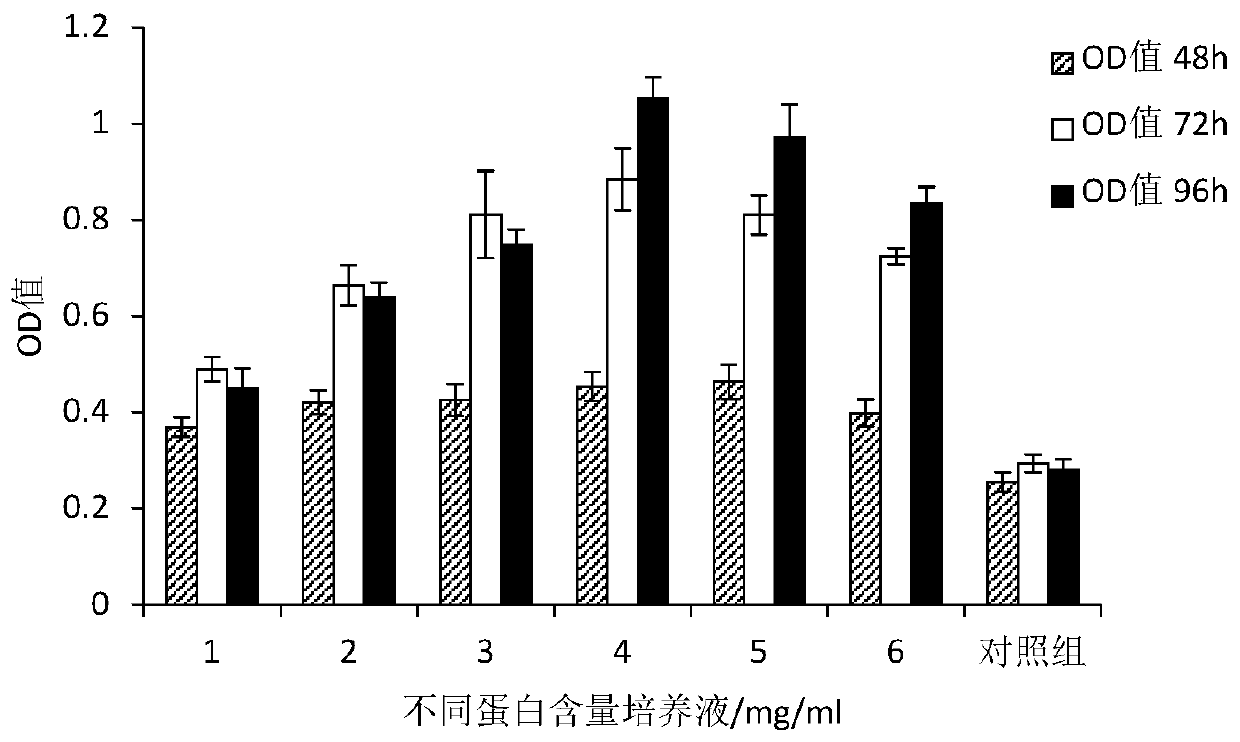
Human umbilical cord mesenchymal stem cell serum-free culture medium and preparation method thereof, and acquisition method of human umbilical cord mesenchymal stem cell serum-free culture solution
A serum-free medium and serum-free culture technology, applied in the field of stem cell culture, can solve the problems of poor stability and slow proliferation, and achieve the effect of improving adherent growth and expansion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] A preparation method for human umbilical cord mesenchymal stem cell serum-free medium, comprising the following specific steps:
[0055] Step 1: Prepare serum-free culture supernatant concentrate
[0056] Human umbilical cord mesenchymal stem cells were inoculated in serum-free medium for culture. When the cell confluence was 80-90%, the supernatant of human umbilical cord mesenchymal stem cells was collected; filtered, concentrated by ultrafiltration, and added with water to determine the total protein concentration. 10mg / mL to obtain a serum-free culture supernatant concentrate.
[0057] Human umbilical cord mesenchymal stem cells were used in step 1 at 5×10 3 ~8×10 3 / cm 2 Inoculate in commercially available serum-free medium.
[0058] In step 1, filter twice, one of which uses a 0.8 μm microfiltration membrane, and the other uses a 0.45 μm microfiltration membrane.
[0059] In step 1, the ultrafiltration concentration adopts an ultrafiltration system with a mol...
Embodiment 1
[0079] A method for preparing human umbilical cord mesenchymal stem cell serum-free medium, specifically comprising the following steps:
[0080] Step 1: Prepare serum-free culture supernatant concentrate
[0081] Human umbilical cord mesenchymal stem cells in 5×10 3 / cm 2 Inoculate in serum-free medium for culture, collect the culture supernatant when the cell fusion degree is 80%, and obtain a supernatant with a volume of 5 L, filter the 5 L supernatant through a plate filter, and filter membrane pore diameters are respectively 0.8μm, 0.45μm; then use cellulose acetate ultrafiltration membrane with a molecular weight cut-off of 3KDa for ultrafiltration and concentration, stop concentration when the concentration reaches 1 / 15 of the original filtered liquid volume, and obtain 305ml of concentrated liquid, use BCA protein reagent The box detects that the protein content of the concentrated solution is 11.2 mg / ml, add 37 ml of water for injection to the concentrated liquid, a...
Embodiment 2
[0089] Prepare a serum-free medium for human umbilical cord mesenchymal stem cells, the method is as follows:
[0090] Step 1: Prepare serum-free culture supernatant concentrate
[0091] Human umbilical cord mesenchymal stem cells in 6×10 3 / cm 2 Inoculate in serum-free medium for culture, collect the culture supernatant when the cell confluence is 85%, obtain a supernatant with a volume of 30L, filter the 30L supernatant through a plate filter in turn, and filter the filter membranes with different pore sizes 0.8μm, 0.45μm; then use cellulose acetate ultrafiltration membrane with a molecular weight cut-off of 3KDa for ultrafiltration and concentration, and the area of a single membrane is 0.5m 2, a total of 6 membrane packs, using the tangential flow process for concentration, the liquid at the permeate end is discarded, and the concentration is stopped when it is concentrated to 1 / 18 of the volume of the original microfiltrate, and the liquid in the ultrafiltration syste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com