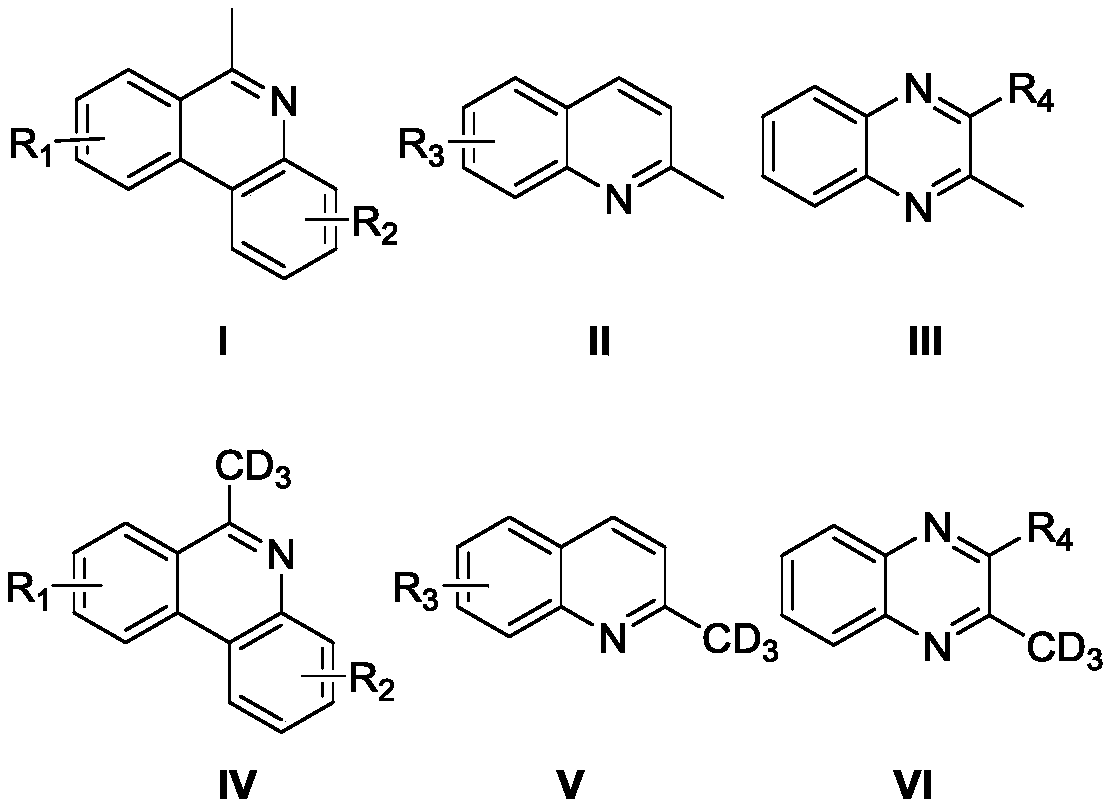
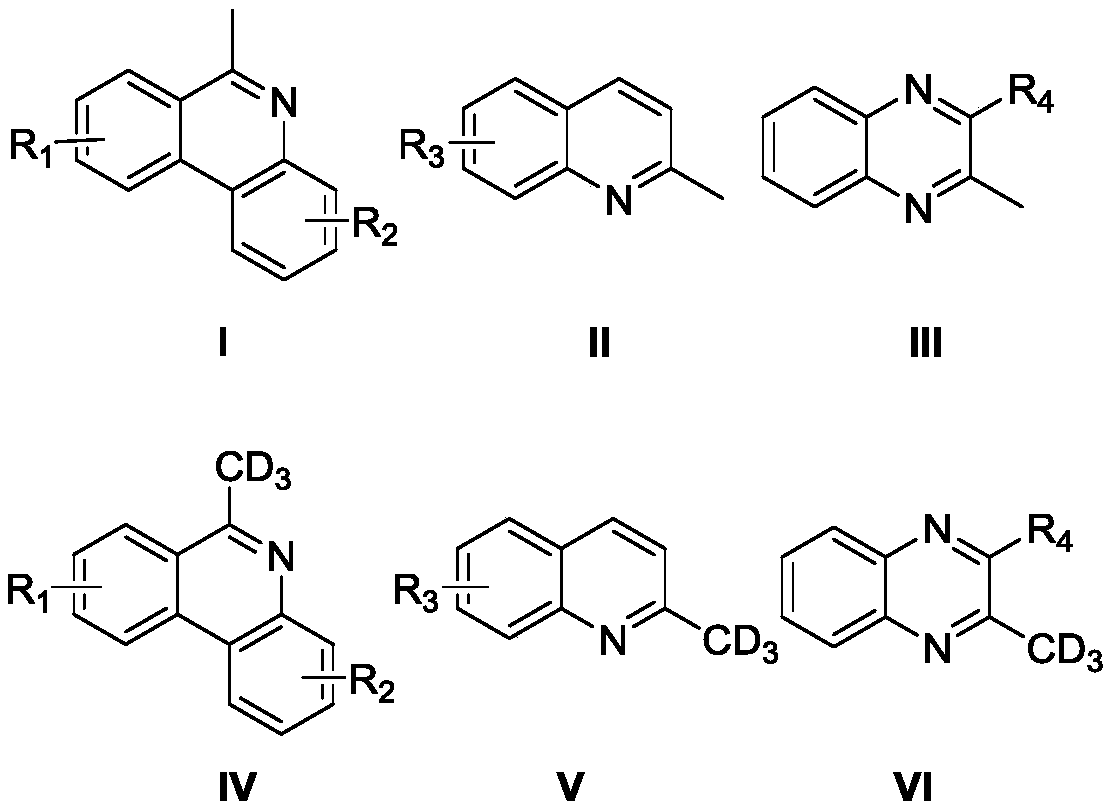
High-selectivity deuteration method of 2-methyl azacyclo compound
A compound and heterocyclic technology, applied in the field of organic compound synthesis, can solve the problems of general chemoselectivity and limited application, and achieve the effects of strong substrate universality, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Add 6-methylphenanthridine (0.3mmol, 58mg), iodobenzene diacetate (0.45mmol, 144.9mg), azobisisobutyronitrile (0.15mmol, 24.6mg) into a dry Schlenk reaction tube, vacuum exchange Nitrogen was blown three times. Under nitrogen protection, deuterium water (300 μL) and N,N-dimethylformamide (3 mL) were added to the reaction tube, and the reaction tube was stirred and reacted at 100° C. for 12 hours. After the reaction, add 10 mL of water to the resulting reaction solution, extract with ether, collect the organic layer, dry with anhydrous sodium sulfate, filter, take the filtrate, evaporate the solvent to obtain 6-(methyl-d3) phenanthridine, produce The rate is 65%, and the deuterium rate is 79%.
[0038] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.54-8.51(m, 1H), 8.47-8.44(m, 1H), 8.14-8.10(m, 1H), 8.09(dd, J1=8.1Hz, J2=1.0Hz, 3H), 7.75(m ,3H),7.70-7.66(m,1H),7.63-7.60(m,1H),7.59-7.55(m,1H),2.97(m,0.3H). 13 C NMR (125 MHz, CDCl 3)δ158.75,143.60,132....
Embodiment 2
[0040]
[0041] Add 8-fluoro-6-methylphenanthridine (0.3mmol, 63.3mg), potassium persulfate (0.45mmol, 121.6mg), azobisisobutyronitrile (0.15mmol, 24.6mg) into a dry Schlenk reaction tube , evacuated and changed nitrogen three times. Under nitrogen protection, deuterium water (300 μL) and N,N-dimethylformamide (3 mL) were added to the reaction tube, and the reaction tube was stirred and reacted at 100° C. for 12 hours. After the reaction, add 10 mL of water to the obtained reaction solution, extract with ether, collect the organic layer, dry with anhydrous sodium sulfate, filter, take the filtrate, evaporate the solvent to obtain 8-fluoro-6-(methyl-d3) Phenanthridine, yield 75%, deuterium rate 34%.
[0042] Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.57(dd,J 1 =9.1Hz,J 2 =5.3Hz,1H), 8.44(d,J=8.2Hz,1H),8.09(dd,J 1 =8.2Hz,J 2 =1.1Hz,1H),7.78(dd,J 1 =9.6 Hz,J 2 =2.5Hz,1H),7.72-7.66(m,1H),7.64-7.58(m,1H),7.58-7.52(m,1H), 2.96-2.94(m,0.13H). 13 C NMR (125MHz, CDC...
Embodiment 3
[0044]
[0045] Add 9-tert-butyl-6-methylphenanthridine (0.3mmol, 74.8mg), cerium ammonium nitrate (0.45mmol, 246.7mg), azobisisobutyronitrile (0.15mmol, 24.6mg) into the dry Schlenk reaction In the tube, evacuate and change nitrogen three times. Under nitrogen protection, add deuterium water (300 μL) and N,N-dimethylformamide (3 mL) into the above reaction tube, and the reaction tube was stirred at 100 ° C for 12 Hour. After the reaction, 10 mL of water was added to the resulting reaction solution, extracted with ether, the organic layer was collected, dried with anhydrous sodium sulfate, filtered, the filtrate was taken, and the solvent was evaporated to obtain 9-tert-butyl-6-(methyl- d3) Phenanthridine, the yield is 69%, and the deuteration rate is 84%. Characterization data: 1 H NMR (500MHz, CDCl 3 )δ8.57-8.50 (m, 2H), 8.18 (d, J = 1.9Hz, 1 H), 8.13 (dd, J 1 =8.2Hz,J 2 =1.0Hz,1H),7.93(dd,J 1 =8.7Hz,J 2 =2.0Hz,1H), 7.72-7.69(m,1H),7.65-7.58(m,1H),3.09-3.06(m,0.18H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com