P-coumaric acid aromatic derivative and preparation method and application thereof
A technology of p-coumaric acid and derivatives, applied in the preparation of sulfonates, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
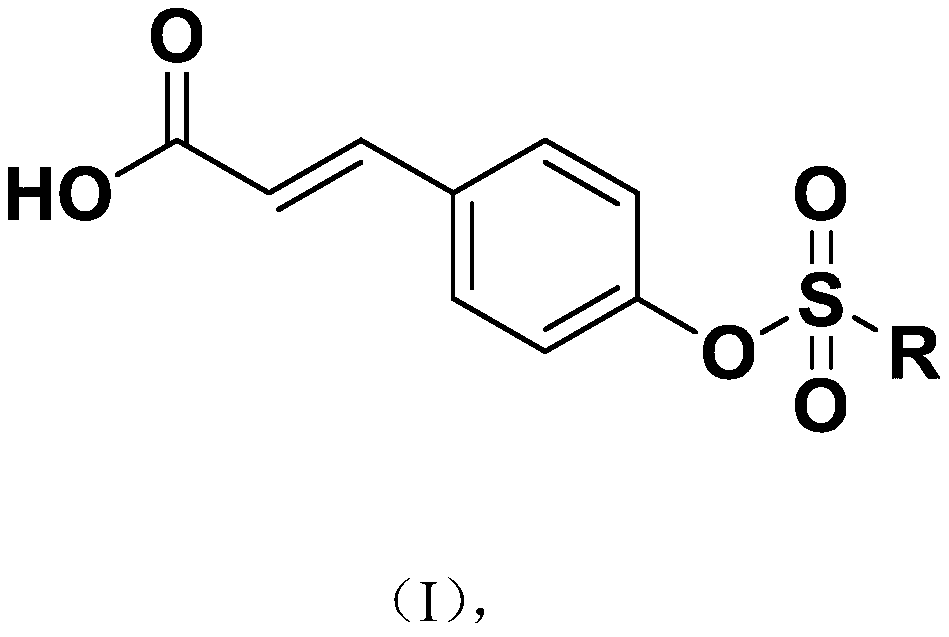
[0026] A class of p-coumaric acid aromatic derivatives of the present invention, the structural formula of the described class of p-coumaric acid aromatic derivatives is shown in formula (I):
[0027]
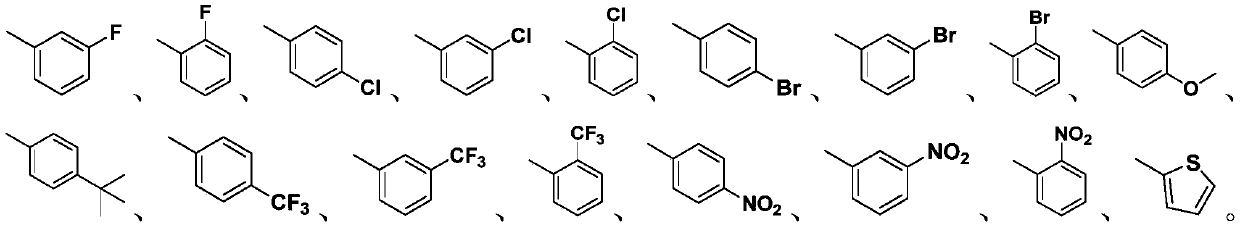
[0028] Wherein, R is selected from:
[0029]
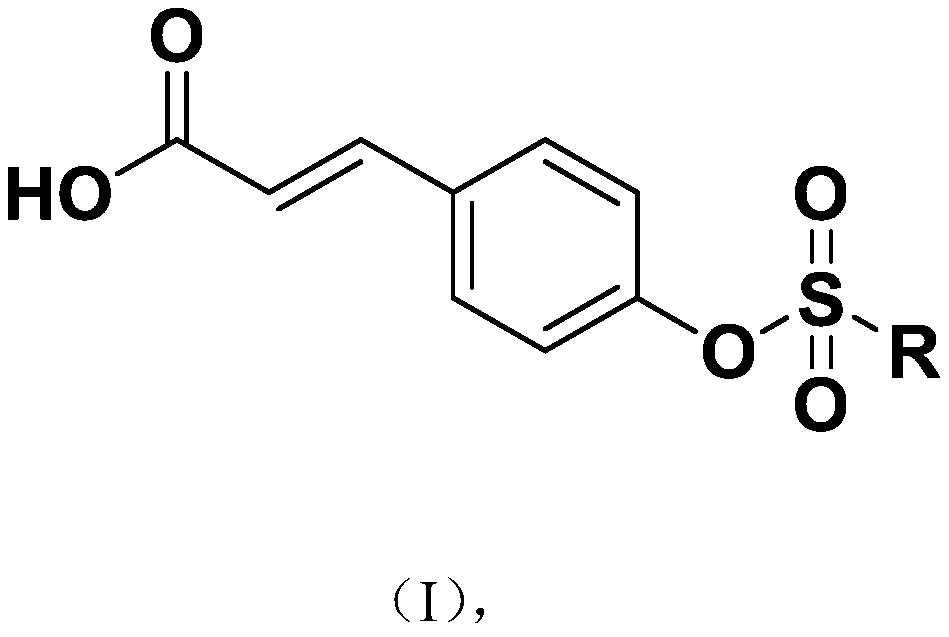
[0030] The synthesis process of a class of p-coumaric acid aromatic derivatives of the present invention has the following general formula:
[0031]
[0032] Wherein, R is selected from:
[0033] The preparation method of a class of p-coumaric acid aromatic derivatives described in the present invention comprises the following steps:
[0034] Dissolve p-coumaric acid in a mixed solution of dichloromethane and N,N-dimethylformamide, add various arylsulfonyl chlorides, stir for 60 minutes under ice bath conditions, add dicyclohexylcarbodiimide and 4-dimethylaminopyridine, moved to room temperature and stirred for 8 hours, followed by thin-layer chromatography. After the reaction, the precipitate was filtered, and the...
Embodiment 2
[0042] The difference between embodiment 2 and embodiment 1 is: the preparation method of a class of p-coumaric acid aromatic derivatives of the present invention, comprises the following steps:
[0043] Dissolve p-coumaric acid in a mixed solution of dichloromethane and N,N-dimethylformamide, add various arylsulfonyl chlorides, stir for 50 minutes under ice bath conditions, add dicyclohexylcarbodiimide and 4-dimethylaminopyridine, moved to room temperature and stirred for 5 hours, followed by thin-layer chromatography. After the reaction, the precipitate was filtered, and the filtrate was extracted with an extractant, washed with saturated sodium chloride solution, and dried with saturated sodium sulfate. The organic solvent was distilled off under reduced pressure, and recrystallized using a mixed solvent of N,N-dimethylformamide and ethanol to obtain a class of aromatic derivatives of p-coumaric acid.
[0044] The extractant is ethyl acetate. The saturated sodium chloride ...
Embodiment 3
[0046] The difference between embodiment 3 and embodiment 1 is:
[0047] The preparation method of a class of p-coumaric acid aromatic derivatives described in the present invention comprises the following steps:
[0048] Dissolve p-coumaric acid in a mixed solution of dichloromethane and N,N-dimethylformamide, add various arylsulfonyl chlorides, stir for 30 minutes under ice bath conditions, add dicyclohexylcarbodiimide and 4-dimethylaminopyridine, moved to room temperature and stirred for 6 hours, and followed the reaction by thin layer chromatography. After the reaction, the precipitate was filtered, and the filtrate was extracted with ethyl acetate, washed with saturated sodium chloride solution, and dried with saturated sodium sulfate. , the organic solvent was distilled off under reduced pressure, and recrystallized using a mixed solvent of N,N-dimethylformamide and ethanol to obtain a class of aromatic derivatives of p-coumaric acid.
[0049] The saturated sodium chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com