Method for preparing p-methoxycinnamic acid
A technology of methoxycinnamic acid and methoxyhalobenzene, which is applied in the field of preparation of intermediate p-methoxycinnamic acid, can solve the problems of difficult industrial production, high price, and difficulty in obtaining, and achieve easy control, Easy to achieve industrial production and reduce pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
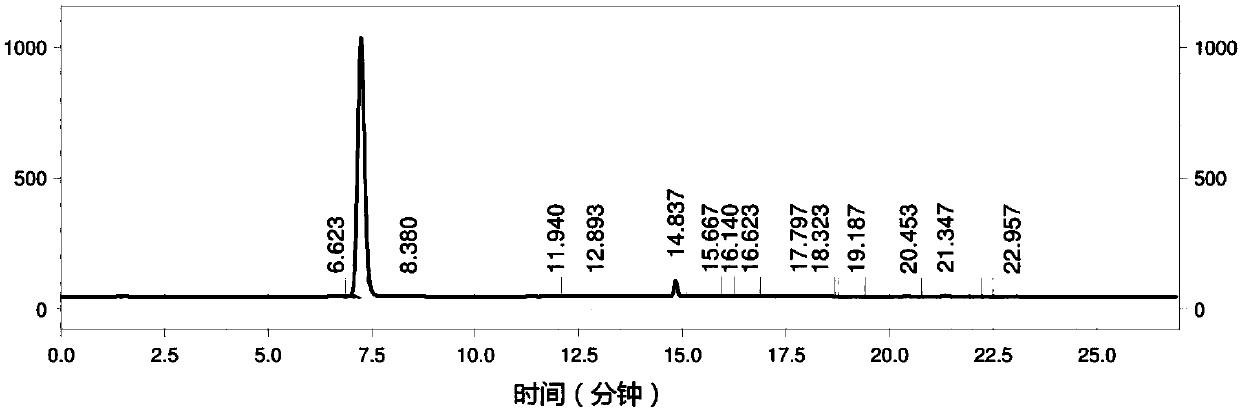
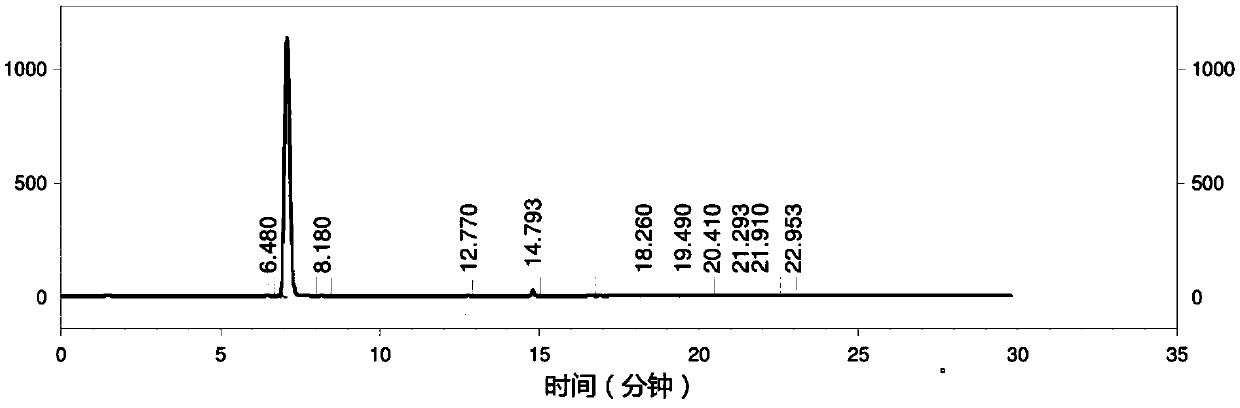
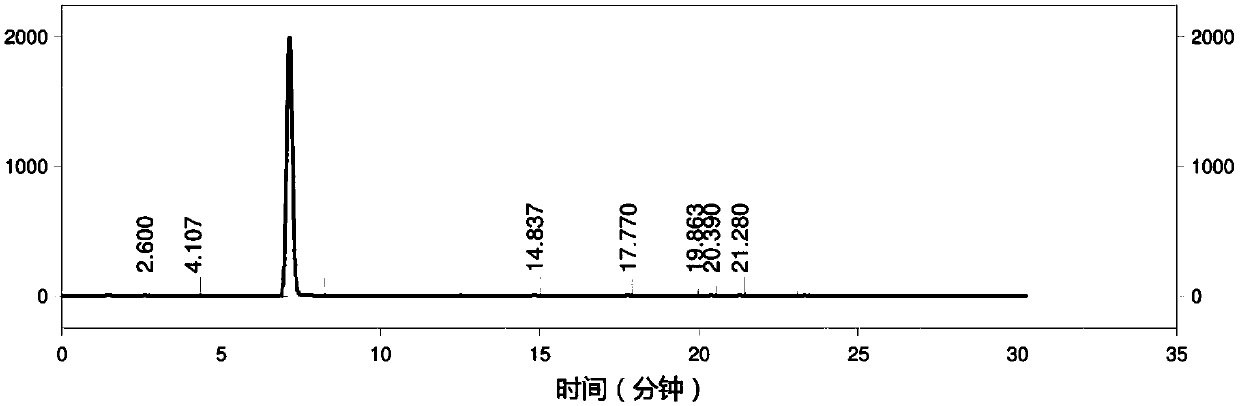
Image
Examples
Embodiment 1
[0069] Salt-forming reaction: Add 19.1g (0.18mol) Na to a 0.5L clean batching kettle 2 CO 3 , 150g tap water, start stirring, slowly add 26g (0.36mol) acrylic acid, after the addition is complete, keep stirring until there is no bubble in the kettle (produce about 7.94gCO 2 gas), clear and transparent.
[0070] Coupling reaction: Add the prepared sodium acrylate solution into a 1L clean autoclave, add 11.87mg Cu 2 (OH) 2 CO 3 (179.33ppm rel.to BA), keep stirring for 10min. In the autoclave, add 19.1g (0.18mol) Na 2 CO 3 , 250g tap water, keep stirring for 10min. Add 56g (0.30mol) 4-methoxybromobenzene (BA) and 3.8mg (17.2×10 -6 mol) BHT, close the feed port of the autoclave, use N 2 Replace 5 times, raise the temperature to 150°C, and keep warm until the pressure no longer increases (the highest pressure is 1.5MPa), which takes about 2 hours. Stirring is accompanied during the reaction, and the stirring speed is maintained at 1200 r / min. Turn off the heating device,...
Embodiment 2
[0074] Salt-forming reaction: add 20.6g (0.19mol) Na to a 0.5L clean batching kettle 2 CO 3 , 150g tap water, start stirring, slowly add 26g (0.36mol) acrylic acid, after the addition is complete, keep stirring until there is no bubble in the kettle and it is clear and transparent.
[0075] Coupling reaction: Add the prepared sodium acrylate solution into a 1L clean autoclave, add 9.97mg Cu 2 (OH) 2 CO 3 (150.80ppm rel.to BA), keep stirring for 10min. Add 15.0g (0.18mol) NaHCO respectively in the autoclave 3 , 250g tap water, keep stirring for 10min. Add 56g (0.30mol) p-bromoanisole (BA) and 4.2mg (23.3×10 -6 mol) BHA, close the feed port of the autoclave, use N 2 Replace 5 times, raise the temperature to 160°C, and keep warm until the pressure no longer increases, about 2 hours. Stirring was accompanied during the reaction, and the stirring speed was kept at 1000 r / min. Turn off the heating device, turn on the condensed water to lower the temperature to 85°C, evacuat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com