Ni-Cu/Al2O3 bimetal catalyst and preparation method thereof, and application of Ni-Cu/Al2O3 bimetal catalyst in preparation of (tert-butylaminoethoxy)ethanol (TBEE)
A bimetallic catalyst, nickel salt technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the loss of active species, waste, increase in reaction cost, etc. problems, to achieve the effect of solving pollution problems, high reactivity, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
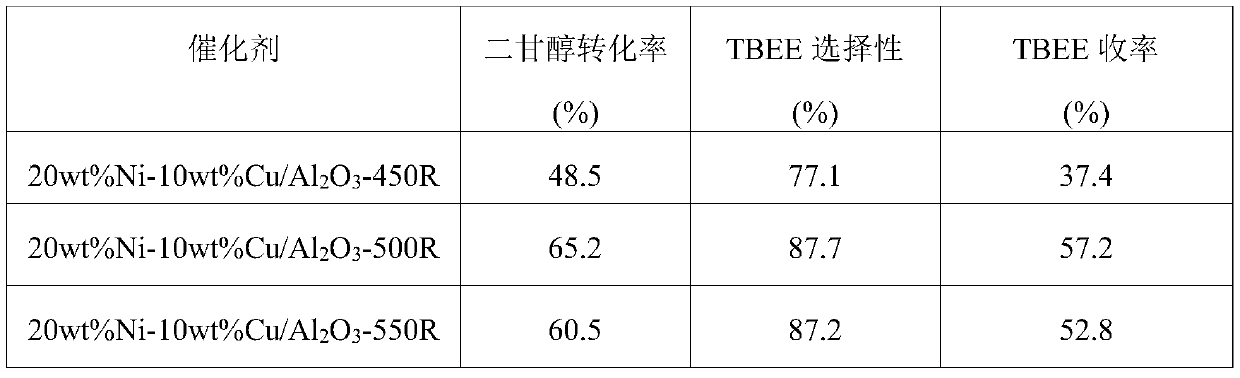
[0017] Weigh 0.991g Ni(NO 3 ) 2 ·6H 2 O and 0.38g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 40 mL of distilled water, and the mixed solution was placed on a rotary stirrer and stirred for 30 min to fully dissolve the solid. Weigh 2g of Al purchased from commercial channels 2 o 3 Carrier (amphoteric metaacid) was added to the above mixed solution, fully immersed for 2h. Afterwards, the excess impregnation solution was heated to 90° C., stirred and evaporated to dryness. The evaporated samples were dried in an oven at 100 °C for 12 h, and then calcined at 500 °C for 4 h in an air atmosphere in a muffle furnace to obtain the precursor of the catalyst. The catalyst precursor that takes by weighing 0.25g is in quartz tube, in 10% (volume fraction) H 2 10wt%Ni-5wt%Cu / Al was obtained by reduction at 500℃ for 2h in Ar atmosphere 2 o 3 catalyst. The catalyst after the reduction is lowered to room temperature, and used in a batch tank reactor to catalyze the reaction of diethylen...
Embodiment 2
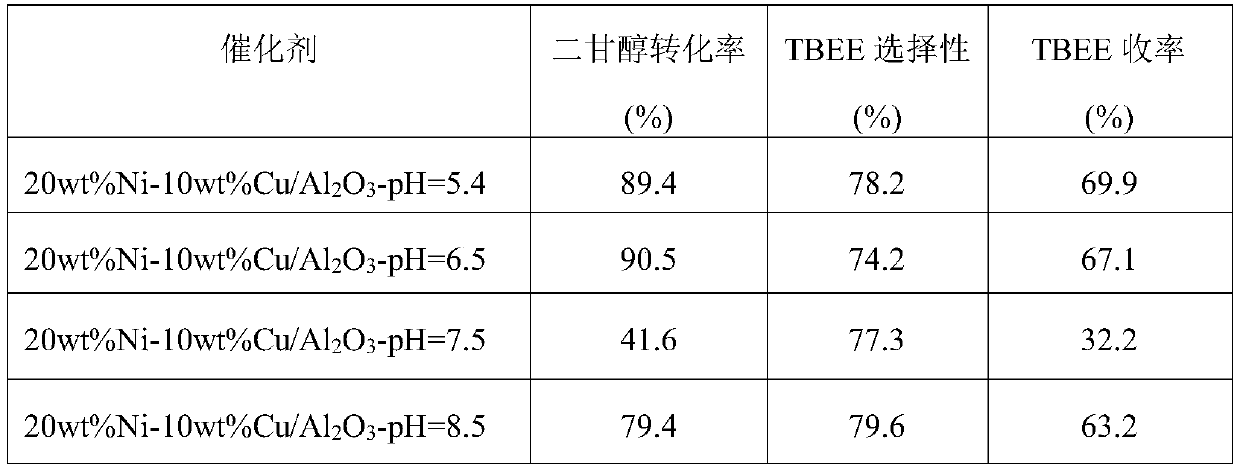
[0019] Weigh 1.486g Ni(NO 3 ) 2 ·6H 2 O and 0.38g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 40 mL of distilled water, and the mixed solution was placed on a rotary stirrer and stirred for 30 min to fully dissolve the solid. Weigh 2g of Al purchased from commercial channels 2 o 3 Carrier (amphoteric metaacid) was added to the above mixed solution, fully immersed for 2h. Afterwards, the excess impregnation solution was heated to 90° C., stirred and evaporated to dryness. The evaporated samples were dried in an oven at 100 °C for 12 h, and then calcined at 500 °C for 4 h in an air atmosphere in a muffle furnace to obtain the precursor of the catalyst. The catalyst precursor powder that takes 0.25g is in quartz tube, in 10% (volume fraction) H 2 15wt%Ni-5wt%Cu / Al was obtained by reduction at 500℃ for 2h in / Ar atmosphere 2 o 3 catalyst. The catalyst after the reduction is lowered to room temperature, and used in a batch tank reactor to catalyze the reaction of diethylene gl...
Embodiment 3
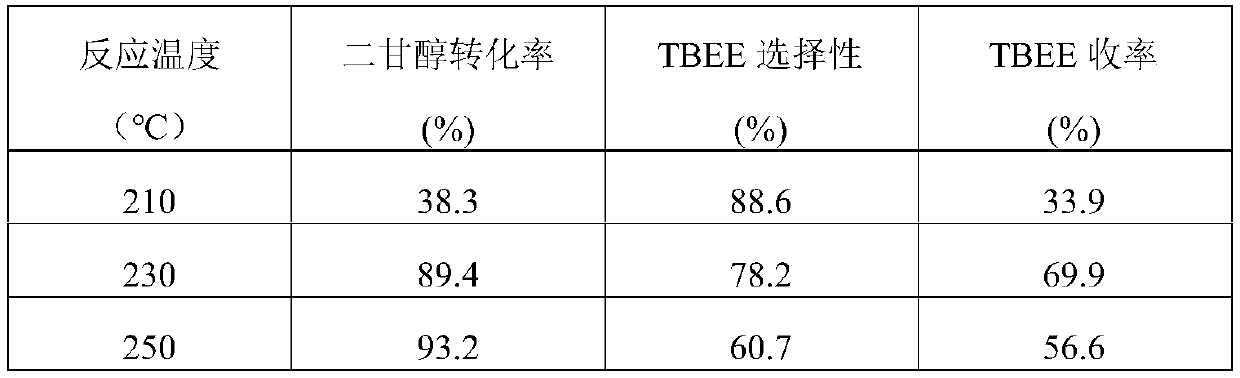
[0021] Weigh 0.495g Ni(NO 3 ) 2 ·6H 2 O and 1.14g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 40 mL of distilled water, and the mixed solution was placed on a rotary stirrer and stirred for 30 min to fully dissolve the solid. Weigh 2g of Al purchased from commercial channels 2 o 3 The carrier (amphoteric metaacid) was added to the above mixed solution, fully immersed for 2h. Afterwards, the excess impregnating solution was heated to 90° C., stirred and evaporated to dryness. The evaporated samples were dried in an oven at 100 °C for 12 h, and then calcined at 500 °C for 4 h in an air atmosphere in a muffle furnace to obtain the precursor of the catalyst. The catalyst precursor powder that takes 0.25g is in quartz tube, in 10% (volume fraction) H 2 Reduction at 500℃ for 2h in / Ar atmosphere to obtain 5wt%Ni-15wt%Cu / Al 2 o3 catalyst. The catalyst after the reduction is lowered to room temperature, and used in a batch tank reactor to catalyze the reaction of diethylene glycol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com