Preparation method of losartan
A technology of losartan and cyano group, applied in the field of preparation of losartan, can solve the problems of inability to eliminate the risk of impurity contamination, violent reaction of the quenching system, difficult control of the quenching process, etc., so as to eliminate the genotoxic impurity nitrosamines. The effect of forming, less reagent dosage, suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
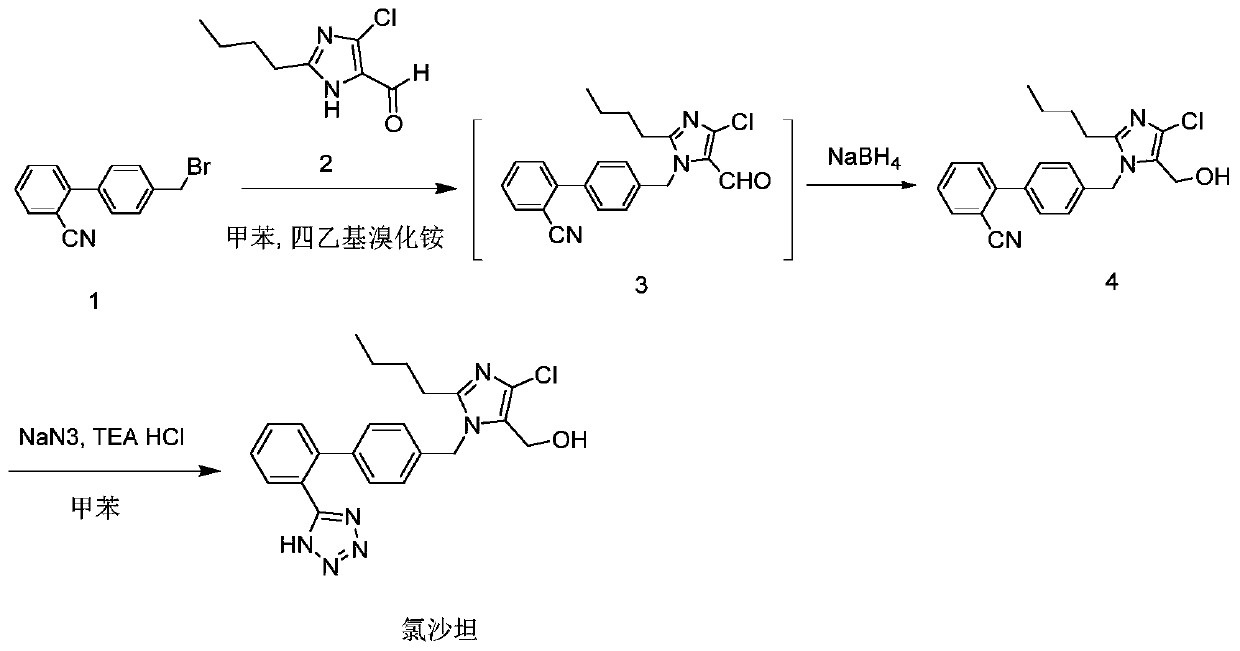
[0052] Preparation of Preparation Example Compound 4
[0053]Put 365g of toluene into the reaction flask, add 56g of compound 1, 33.6g of sodium carbonate and 5.6g of tetraethylammonium bromide under slow stirring, speed up the stirring and raise the temperature to 90-95°C. Control the temperature at 90-95°C, add 39.2g of compound 2 in 142g toluene solution dropwise, control the dropping time for 3-4h, keep warm for 4-5h after the dropwise addition is completed, add 140g of water after the reaction is completed, separate layers, and the toluene layer Add 1g of tetraethylammonium bromide, control the temperature at 20-25°C, add dropwise the pre-prepared mixed solution of sodium borohydride and sodium hydroxide (9g sodium borohydride, 5.6g sodium hydroxide, 24.5g water), dropwise Complete the heat preservation reaction at 20-25°C for 4 hours, then raise the temperature to 80-85°C heat preservation reaction, add 105g water after the reaction, control the temperature at 80-85°C he...
Embodiment 1
[0054] The preparation of embodiment 1 losartan
[0055] Put 40g of toluene into the four-necked bottle, add 20g of compound 4, 10.3g of sodium azide and 18g of triethylamine hydrochloride under slow stirring, after feeding is completed, heat up to 90-95°C, keep the temperature for 35-45 hours, and react After completion, add 40g of water, lower the temperature to 60-70°C, let stand for stratification, separate the middle layer material liquid, add 40ml of n-butanol to dissolve, wash twice with 20g of saturated sodium chloride aqueous solution, add 0.4g of triphenylphosphine and 0.8g of activated carbon, stirred and heated to 50-60°C for decolorization for 2 hours. Filtrate hot, rinse the filter cake with 10ml n-butanol, combine the filtrates, control the temperature at 50-80°C, vacuum dehydration above the vacuum degree of 0.08MPa, add 7.7g liquid caustic soda (concentrated hydrogen with a mass concentration of about 50%) Sodium oxide solution) and 160g water, stir to dissol...
Embodiment 2
[0056] The preparation of embodiment 2 losartan
[0057] Put 40g of toluene into the four-necked bottle, add 20g of compound 4, 10.3g of sodium azide and 18g of triethylamine hydrochloride under slow stirring, after feeding is completed, heat up to 90-95°C, keep the temperature for 35-45 hours, and react After completion, add 40g of water, lower the temperature to 60-70°C, let stand for stratification, separate the middle layer material liquid, add 40ml of n-butanol to dissolve, wash twice with 20g of saturated sodium chloride aqueous solution, add 1.0g of triphenylphosphine and 0.8g of activated carbon, stirred and heated to 50-60°C for decolorization for 2 hours. Filtrate hot, rinse the filter cake with 10ml n-butanol, combine the filtrates, control the temperature at 50-80°C, vacuum dehydration above the vacuum degree of 0.08MPa, add 7.7g liquid caustic soda (concentrated hydrogen with a mass concentration of about 50%) Sodium oxide solution) and 160g water, stir to dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com