Hypoxia sensitive response type chitosan-nitroimidazole graft, and preparation method and application thereof
A technology of nitroimidazole and chitosan, which is applied in the application field of chitosan nitroimidazole grafts as hypoxia-sensitive responsive drug release carriers, can solve problems such as difficult to achieve rapid and effective drug release, and achieve improved Effects of drug release efficiency, improvement of drug efficacy, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
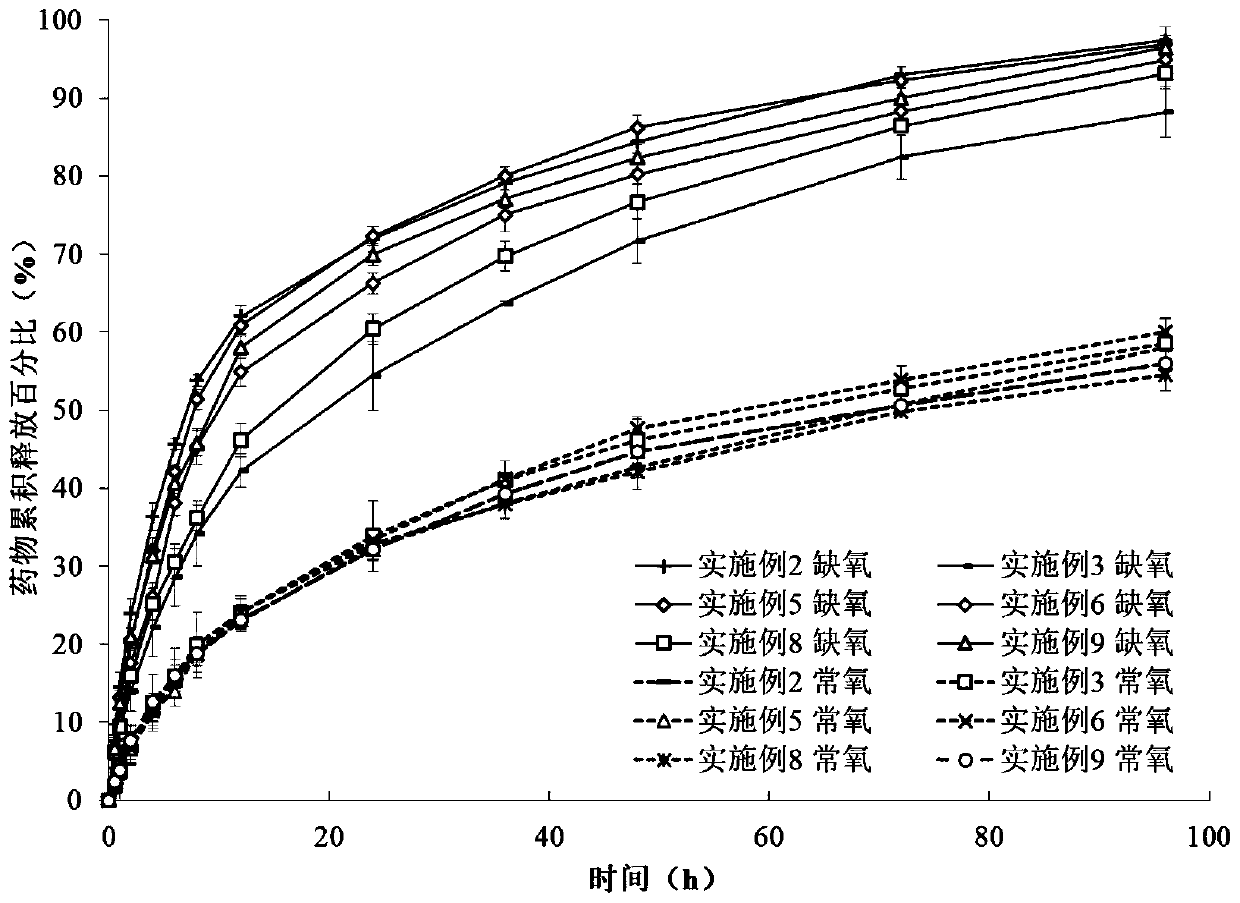
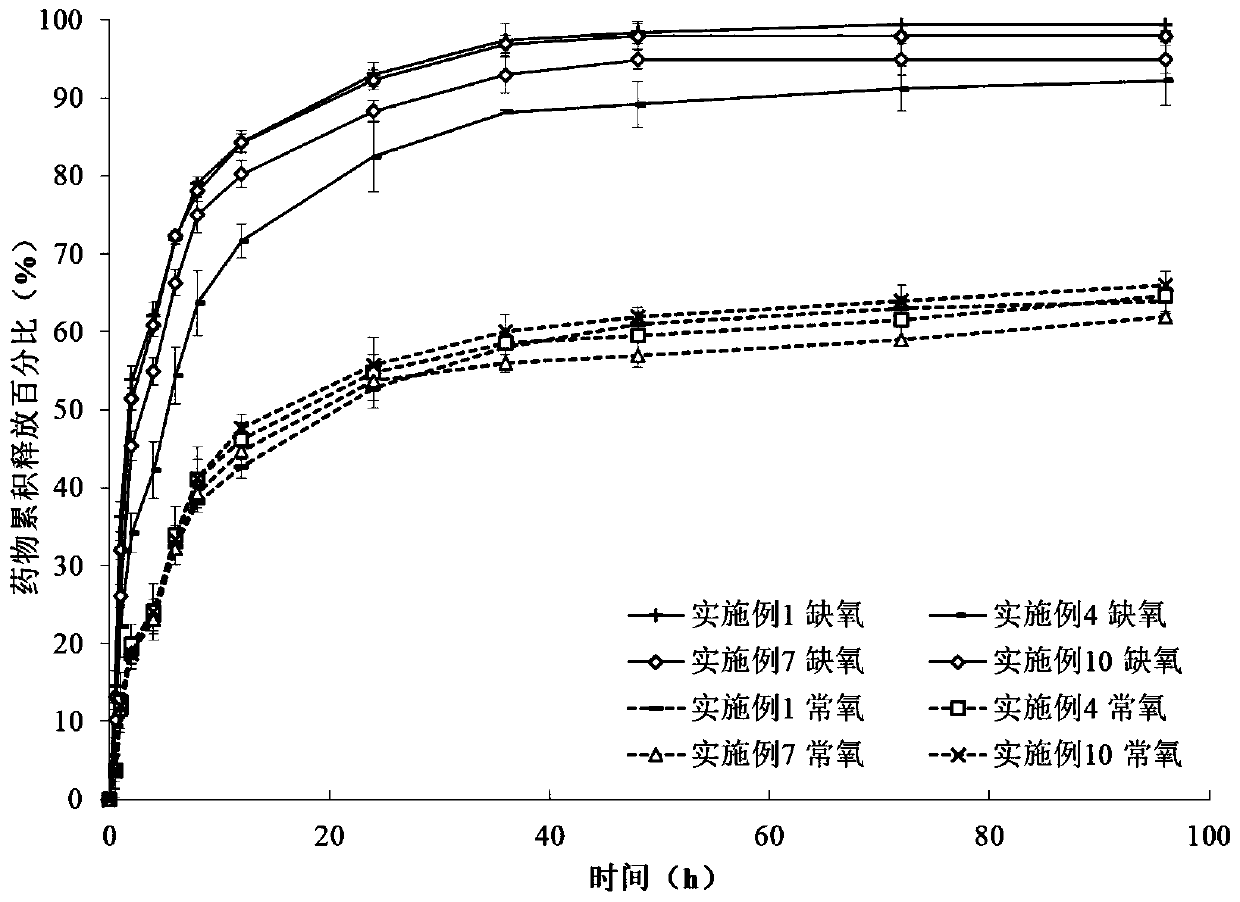
Examples
Embodiment 1
[0029]Accurately weigh 64.8mg 2-nitroimidazole, 39.6mg potassium carbonate and 128.5mg N-Boc-6-bromohexylamine into a 50mL dry round bottom flask, add 10mL dehydrated N,N-dimethylformamide ( N, N-dimethylformamide, DMF), reacted at 80°C for 4h in a constant temperature heating magnetic stirrer. After the reaction, centrifuge to take the supernatant of the reaction solution, add 100mL deionized water and 20mL ethyl acetate, shake at room temperature on an air bath oscillator for 5min, and let it stand for 30min. After the liquid is separated, take the upper layer of ethyl acetate and place it in a Round-bottomed flask, evaporating in a water bath at 40°C on a rotary evaporator until the solution in the flask is completely removed. Add 30mL of DMF, 73.4mg of disuccinimidyl carbonate (N,N'-Disuccinimidyl Carbonate, DSC), 186.8mg of chitosan (Mw=2000Da) and 30mL of deionized water into the flask, and heat it in a magnetic stirrer at a constant temperature of 40°C After 24 hours o...
Embodiment 2
[0031] Accurately weigh 64.8mg of 2-nitroimidazole, 79.2mg of potassium carbonate and 160.6mg of N-Boc-6-bromohexylamine into a 50mL dry round bottom flask, add 20mL of dehydrated DMF, and heat in a magnetic stirrer at a constant temperature for 80 ℃ reaction 4h. After the reaction, centrifuge to take the supernatant of the reaction solution, add 100mL deionized water and 20mL ethyl acetate, shake at room temperature on an air bath oscillator for 5min, and let it stand for 30min. After the liquid is separated, take the upper layer of ethyl acetate and place it in a Round-bottomed flask, evaporating in a water bath at 40°C on a rotary evaporator until the solution in the flask is completely removed. Add 30mL DMF, 146.8mg DSC, 373.6mg chitosan (Mw=5000Da) and 30mL deionized water into the flask, and react in a constant temperature heating magnetic stirrer at 40°C for 24h to obtain a reaction product. The reaction product was placed in a dialysis bag (MWCO 3500Da) and dialyzed f...
Embodiment 3
[0033] Accurately weigh 64.8mg 2-nitroimidazole, 158.2mg potassium carbonate and 240.9mg N-Boc-6-bromohexylamine into a 50mL dry round bottom flask, add 20mL dehydrated DMF, and heat in a magnetic stirrer at a constant temperature for 80 ℃ reaction 4h. After the reaction, centrifuge to take the supernatant of the reaction solution, add 100mL deionized water and 20mL ethyl acetate, shake at room temperature on an air bath oscillator for 5min, and let it stand for 30min. After the liquid is separated, take the upper layer of ethyl acetate and place it in a Round-bottomed flask, evaporating in a water bath at 40°C on a rotary evaporator until the solution in the flask is completely removed. Add 30mL DMF, 220.2mg DSC, 934.1mg chitosan (Mw=20000Da) and 30mL deionized water into the flask, and react in a constant temperature heating magnetic stirrer at 40°C for 24h to obtain a reaction product. The reaction product was placed in a dialysis bag (MWCO 3500Da) and dialyzed for 48 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical micelle concentration (mass) | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com