Method for synthesizing asymmetric cyanoalkyl disulfide
A technology of cyanoalkyl disulfide and cyanoalkyl disulfide, which is applied in the field of synthesis of asymmetric cyanoalkyl disulfide, which can solve the problem of environmentally unfriendly irritating odor, difficulty in large-scale production, and environmental pollution and other issues, to achieve the effect of low price, easy access, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
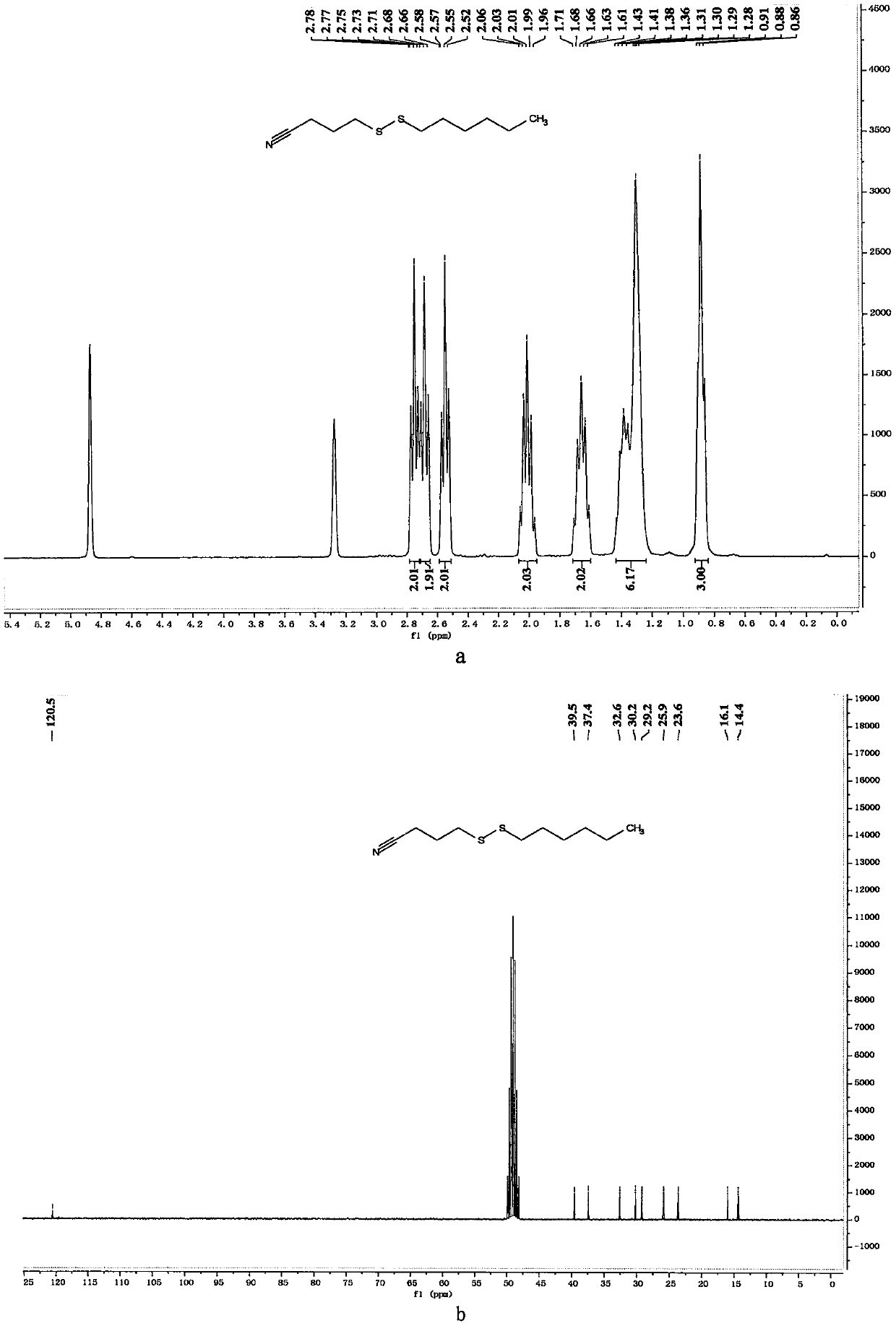
[0056] Preparation of 4-(hexyldithio)butyronitrile
[0057]
[0058] In a 10mL reaction bottle equipped with a magnet, add 4-cyanobutyl sodium thiosulfate (203mg, 1mmol, 1.25equiv.), thiourea (73mg, 0.96mmol, 1.20equiv.), sodium carbonate (102mg , 0.96mmol, 1.20equiv.), sodium dodecylbenzenesulfonate (34.8mg, 0.1mmol, 0.125equiv.), 1-bromohexane (132mg, 0.8mmol, 1equiv.) and water (1mL), in Under the protection of nitrogen, the reaction was stirred at 80° C. for 7 hours. The reaction was cooled to room temperature, 5 mL of water was added, the mixture was extracted with ethyl acetate (15 mL×3), the organic phases were combined, dried with 4 g of anhydrous magnesium sulfate, filtered through filter paper, and vacuum reduced (vacuum degree 95 mmHg, heating temperature 48 ° C) The solvent was removed, and 4-(hexyldithio)butyronitrile was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 20:1). Yield: 83%; 1 H NMR (300MHz, CD ...
Embodiment 2
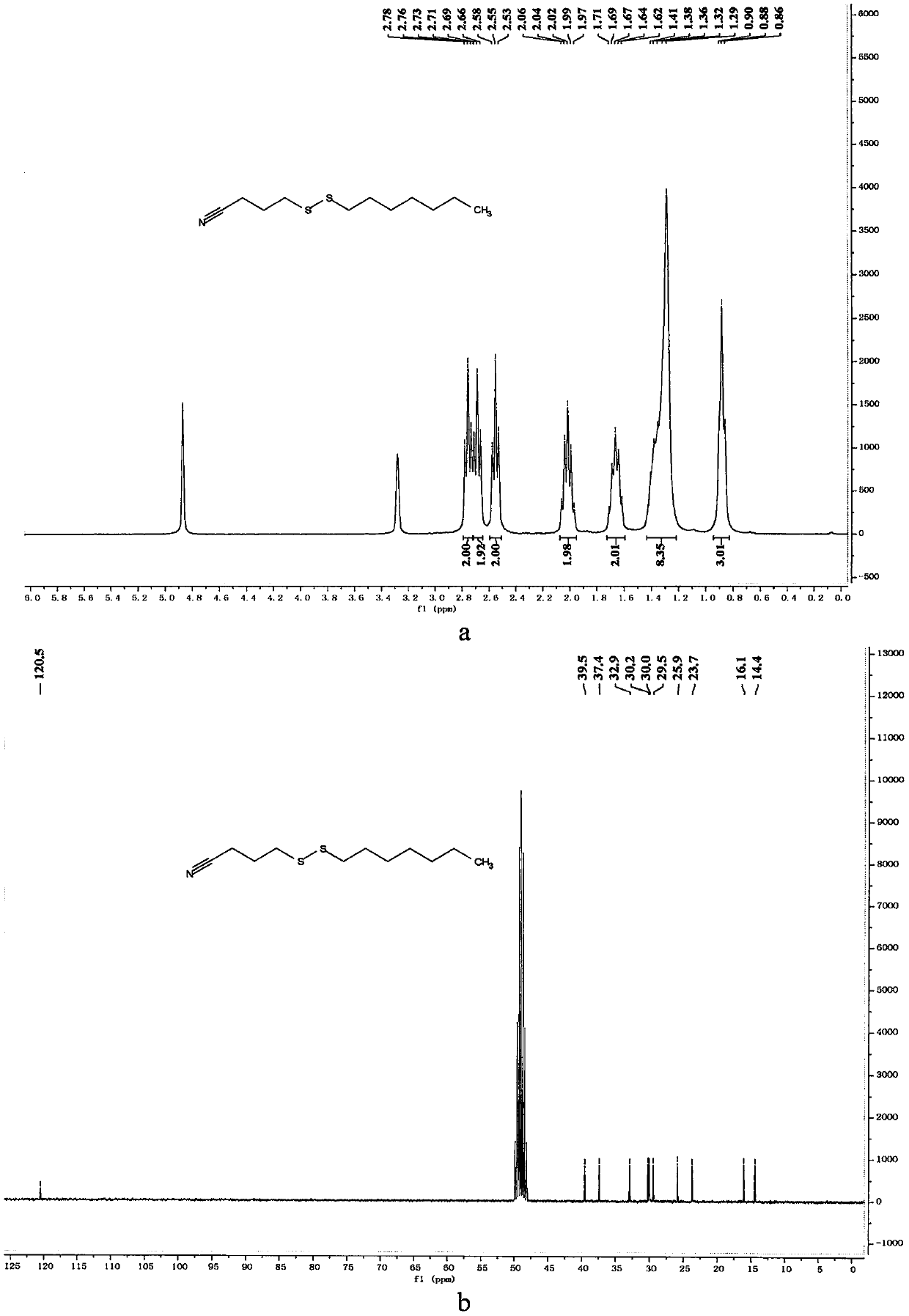
[0060] Preparation of 4-(hexyldithio)butyronitrile
[0061]
[0062] In a 10mL reaction bottle equipped with a magnet, add 4-cyanobutyl sodium thiosulfate (203mg, 1mmol, 1.25equiv.), thiourea (73mg, 0.96mmol, 1.20equiv.), sodium carbonate (102mg , 0.96mmol, 1.20equiv.), sodium dodecylbenzenesulfonate (34.8mg, 0.1mmol, 0.125equiv.), 1-iodohexane (169mg, 0.8mmol, 1equiv.) and water (1mL), in Under the protection of nitrogen, the reaction was stirred at 80° C. for 7 hours. The reaction was cooled to room temperature, 5 mL of water was added, the mixture was extracted with ethyl acetate (15 mL×3), the organic phases were combined, dried with 4 g of anhydrous magnesium sulfate, filtered through filter paper, and vacuum reduced (vacuum degree 95 mmHg, heating temperature 48 ° C) The solvent was removed, and 4-(hexyldithio)butyronitrile was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 20:1). Yield: 89%; 1 H NMR, 13 C NMR an...
Embodiment 3
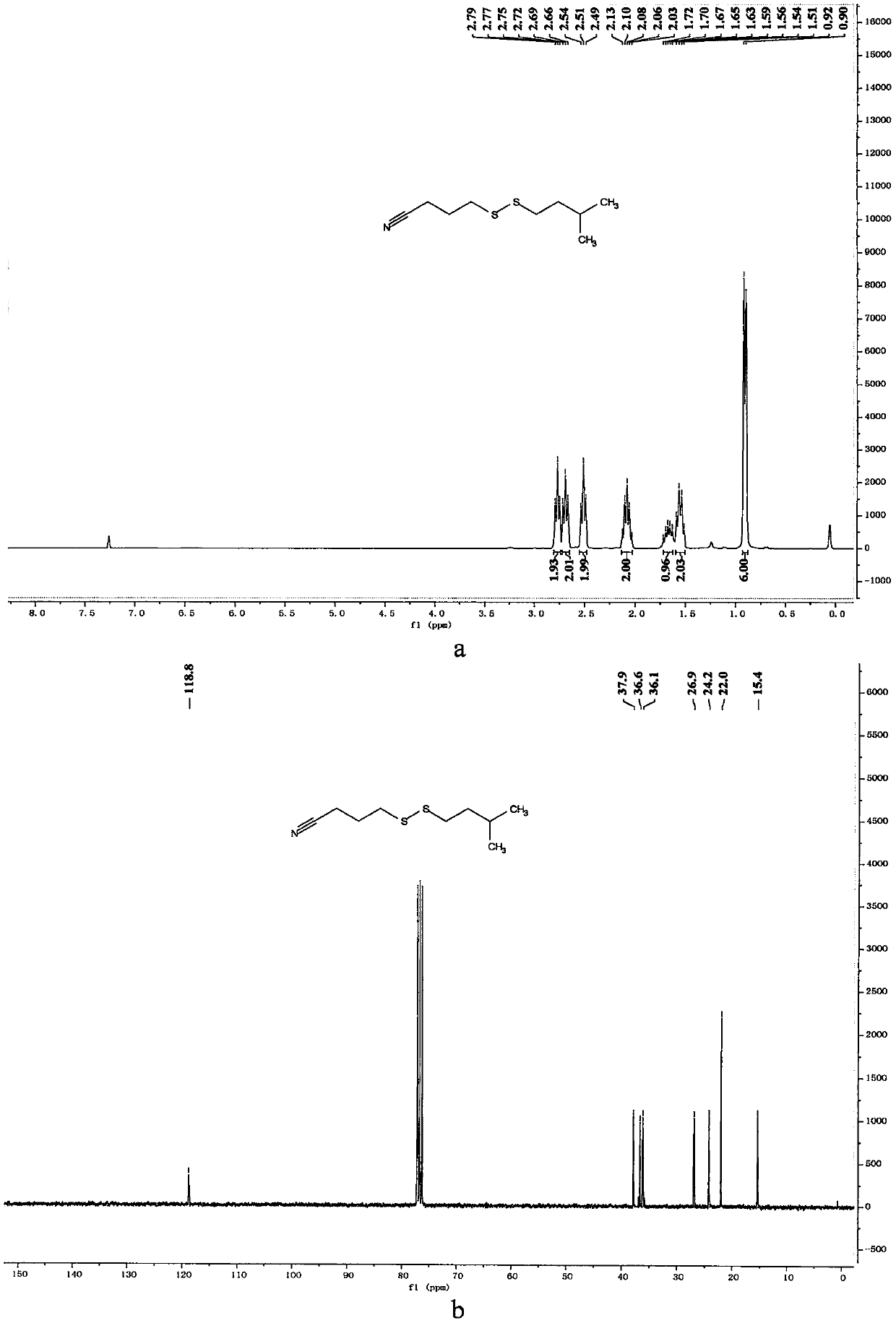
[0065] Preparation of 4-(heptyldithio)butyronitrile
[0066]
[0067] In a 10mL reaction bottle equipped with a magnet, add 4-cyanobutyl sodium thiosulfate (203mg, 1mmol, 1.25equiv.), thiourea (73mg, 0.96mmol, 1.20equiv.), sodium carbonate (102mg , 0.96mmol, 1.20equiv.), sodium dodecylbenzenesulfonate (34.8mg, 0.1mmol, 0.125equiv.), 1-bromoheptane (142mg, 0.8mmol, 1equiv.) and water (1mL), in Under the protection of nitrogen, the reaction was stirred at 80° C. for 7 hours. The reaction was cooled to room temperature, 5 mL of water was added, the mixture was extracted with ethyl acetate (15 mL×3), the organic phases were combined, dried with 4 g of anhydrous magnesium sulfate, filtered through filter paper, and vacuum reduced (vacuum degree 95 mmHg, heating temperature 48 ° C) The solvent was removed, and 4-(heptyldithio)butyronitrile was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 20:1). Yield: 76%; 1 H NMR (300MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com