Preparation method of human papillomavirus and heat shock protein recombinant protein
A technology of human papillomavirus and heat shock protein, which is applied in the field of bioengineering, can solve problems such as the difficulty of expressing and purifying HPVE6, the inability to guarantee safety, and the impact on the approval of new drugs, etc., to achieve improved renaturation rate and purity, low cost, and easy operation convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of the optimized codon gene of embodiment 1 human papillomavirus (HPV) 16 type E6E7HSP70 fusion protein
[0051] According to the obtained HPV16 early gene E6 and E7 encoded polypeptide amino acid sequence and mycobacterial heat shock protein 70 gene, adopt HPV16 early gene E6, E7 and mycobacterium heat shock protein 70 protein sequence combination design as shown in SEQID NO:2 indicated fusion protein.
[0052] Utilizing the degeneracy and preference of the gene, the optimized codon nucleotide sequence was designed under the premise that the amino acid sequence of the encoded product protein remained unchanged. The inventor named the sequence E6E7HSP70. Experimental testing proved that the optimized gene It is suitable for expressing E6E7HSP70 fusion protein in Escherichia coli, and the optimized gene sequence is shown in SEQ ID NO:1.
Embodiment 2
[0053] Embodiment 2 Construction of prokaryotic expression recombinant plasmid
[0054] The synthesized and cloned E6E7HSP70 optimized gene was digested with restriction enzymes, inserted between the NcoI and BamH I restriction sites of the Escherichia coli expression vector pET28a, identified by NcoI and BamH I restriction and sequenced and screened to obtain the correct inserted prokaryotic Expression of recombinant plasmid pET28a-E6E7HSP70.
Embodiment 3
[0055] The construction of embodiment 3 escherichia coli engineering bacteria (recombinant bacteria)
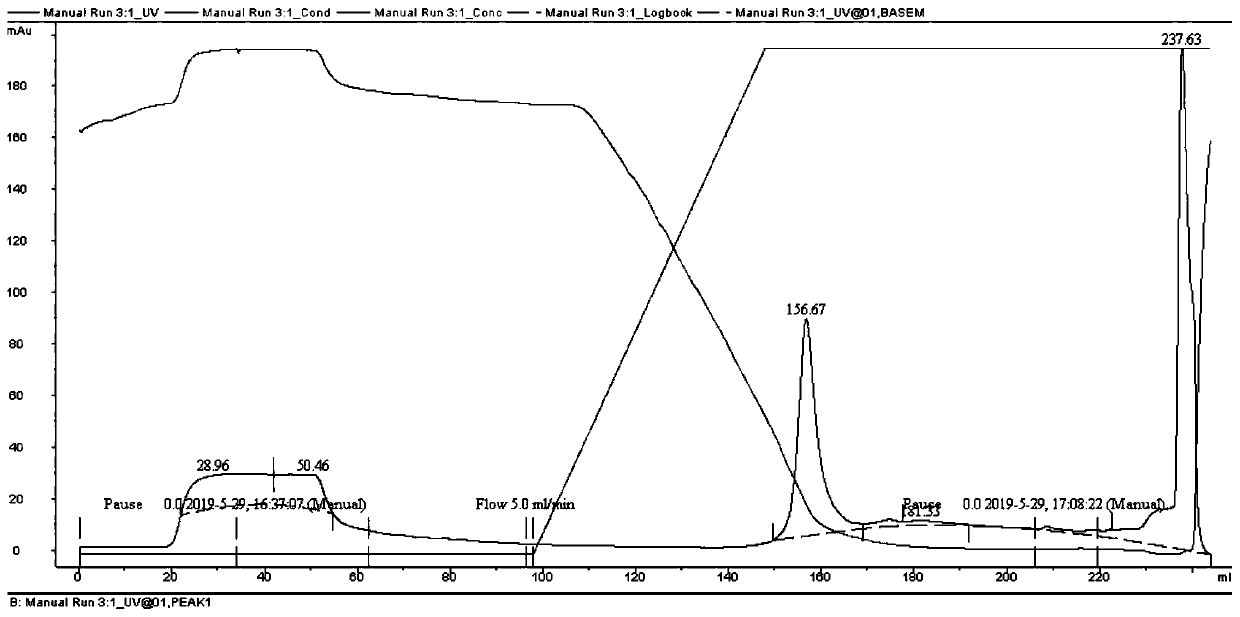
[0056] The recombinant plasmid pET28a-E6E7HSP70 obtained in Example 2 was used to transform BL21(DE3) Escherichia coli, and cultured overnight at 37° C. after spreading on a plate. Picking a single spot in LB medium (see references for the medium recipe: Sambrook J, Fritsch EF, and Manny Artis T, Molecular Cloning Experiment Guide. 1992. Second Edition (Beijing): Science Press : page 908), when OD 600 = 0.6, adding IPTG with a final concentration of 0.4mM to induce expression for 2, 3, and 4 hours respectively, and taking an appropriate amount of bacteria for SDS-PAGE gel electrophoresis analysis, the results are shown in figure 1 , there is a newly added protein band at the molecular weight of about 49KD, this protein band (recombinant protein) accounts for about 20% of the total protein output of the whole bacteria, and the positive clone is preserved as the original bacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com