Preparation method of medical intermediate, namely N-Boc-trans-4-methyl-L-proline methyl ester
A technology of proline methyl ester and n-boc-, which is applied in the field of biomedicine, can solve the problems of poor stereoselectivity, and achieve the effects of shortening the reaction cycle, being environmentally friendly, and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
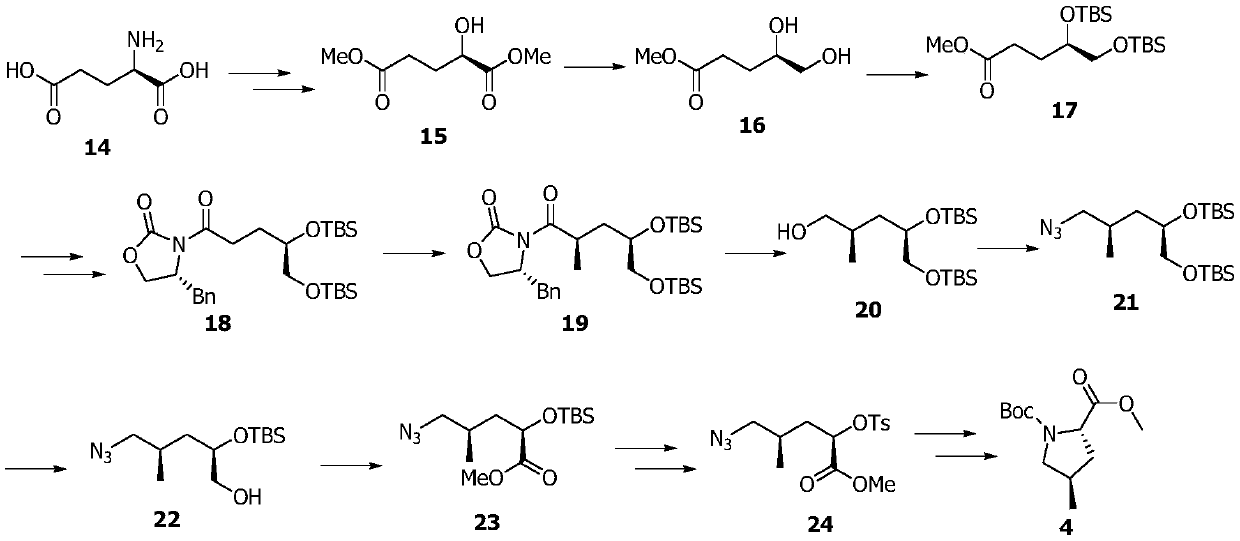
[0068] Embodiment 1: the synthesis of compound 15
[0069]
[0070] Firstly, compound 15 was prepared according to the literature (Tetrahedron Letters, 2017, 58, 3966-3969).
[0071] Compound D-glutamic acid (60g, 407.8mmol) was dissolved in concentrated hydrochloric acid (120mL) and water (60mL), cooled to -10°C, an aqueous solution (120mL) of sodium nitrite (42g, 608.7mmol) was added, slowly After rising to room temperature and reacting for 18 hours, it was concentrated under reduced pressure, the solid residue was extracted with ethyl acetate, filtered, and the filtrate was concentrated under reduced pressure to obtain an intermediate.
[0072] Dissolve the above intermediate in methanol (300 mL), add concentrated hydrochloric acid (0.3 mL), heat to reflux for 12 h, add solid sodium bicarbonate to quench the reaction, concentrate under reduced pressure to obtain the crude product, and use petroleum ether: ethyl acetate = 1 : 1 is the eluent flash column chromatography t...
Embodiment 2
[0073] Embodiment 2: the synthesis of compound 17
[0074] Compound 15 (40g, 227mmol) was dissolved in anhydrous THF (500mL), cooled to 0 degrees, borane dimethyl sulfide complex (10M, 23mL) was added, and sodium borohydride solid (0.5g) was added after 1h , reacted for another 1 h, quenched the reaction with methanol, and concentrated under reduced pressure to obtain the intermediate (R)-methyl 4,5-dihydroxypentanoate.
[0075] The above intermediate (R)-methyl 4,5-dihydroxypentanoate was dissolved in DMF (100mL), imidazole (46g, 681mmol) and tert-butyldimethylsilyl chloride (68g, 454mmol) were added, and the Reaction overnight, add water (600mL) to quench the reaction, extract three times with dichloromethane (300mL), combine the organic phase with 1M KHSO 4 Solution (200mL), washed with water (200mL), washed with saturated brine (200mL), separated, the organic phase was dried by adding anhydrous sodium sulfate, concentrated under reduced pressure, and eluted with petroleum...
Embodiment 3
[0076] Embodiment 3: the synthesis of compound 18
[0077] Compound 17 (20g, 53mmol) was dissolved in methanol / tetrahydrofuran / water mixed solvent (1:1:1, 150mL), and lithium hydroxide monohydrate solid (11g, 265mmol) was added under cooling in an ice-water bath. After 30min, it was raised to room temperature and stirred for reaction 3h, cooled in an ice-water bath, added dilute hydrochloric acid to acidify the solution to PH = 2, extracted three times with ethyl acetate (150mL), combined organic phases were washed with water (100mL), washed with saturated brine (150mL), separated, and the organic phase was added without Dry over sodium sulfate and concentrate under reduced pressure to obtain the intermediate acid (R)-4,5-bis(tert-butyldimethylsilyloxy)pentanoic acid.
[0078] Dissolve the above intermediate acid (R)-4,5-bis(tert-butyldimethylsilyloxy)pentanoic acid in anhydrous THF (200mL), add triethylamine (22.1mL, 159mmol ) and trimethylacetyl chloride (6.5mL, 53mmol), re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com