Self-repairable and recoverable organosilicon elastomer based on imine bond, and preparation method thereof
A self-healing, silicone technology, applied in the field of flexible materials, can solve the problems of poor recyclability and insufficient tensile strength, and achieve the effect of low cost, universal applicability and high recyclability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
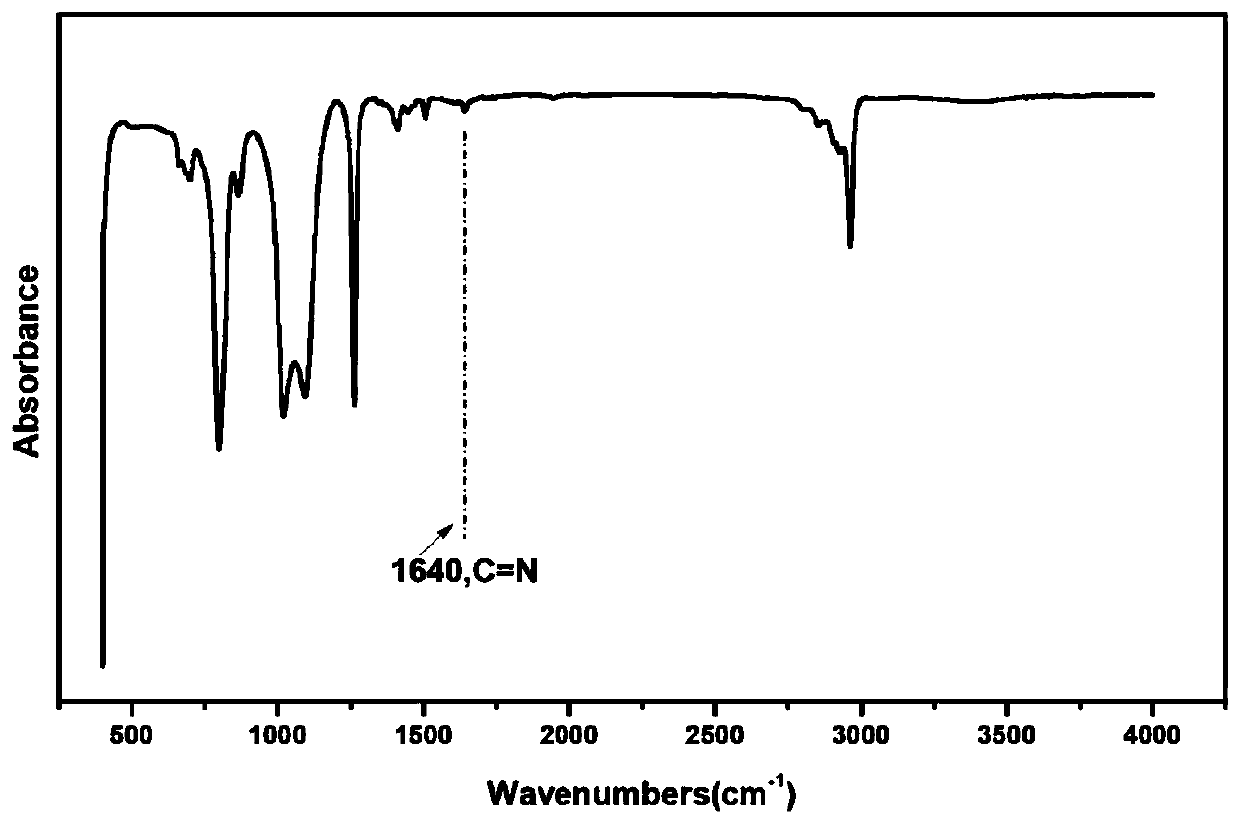
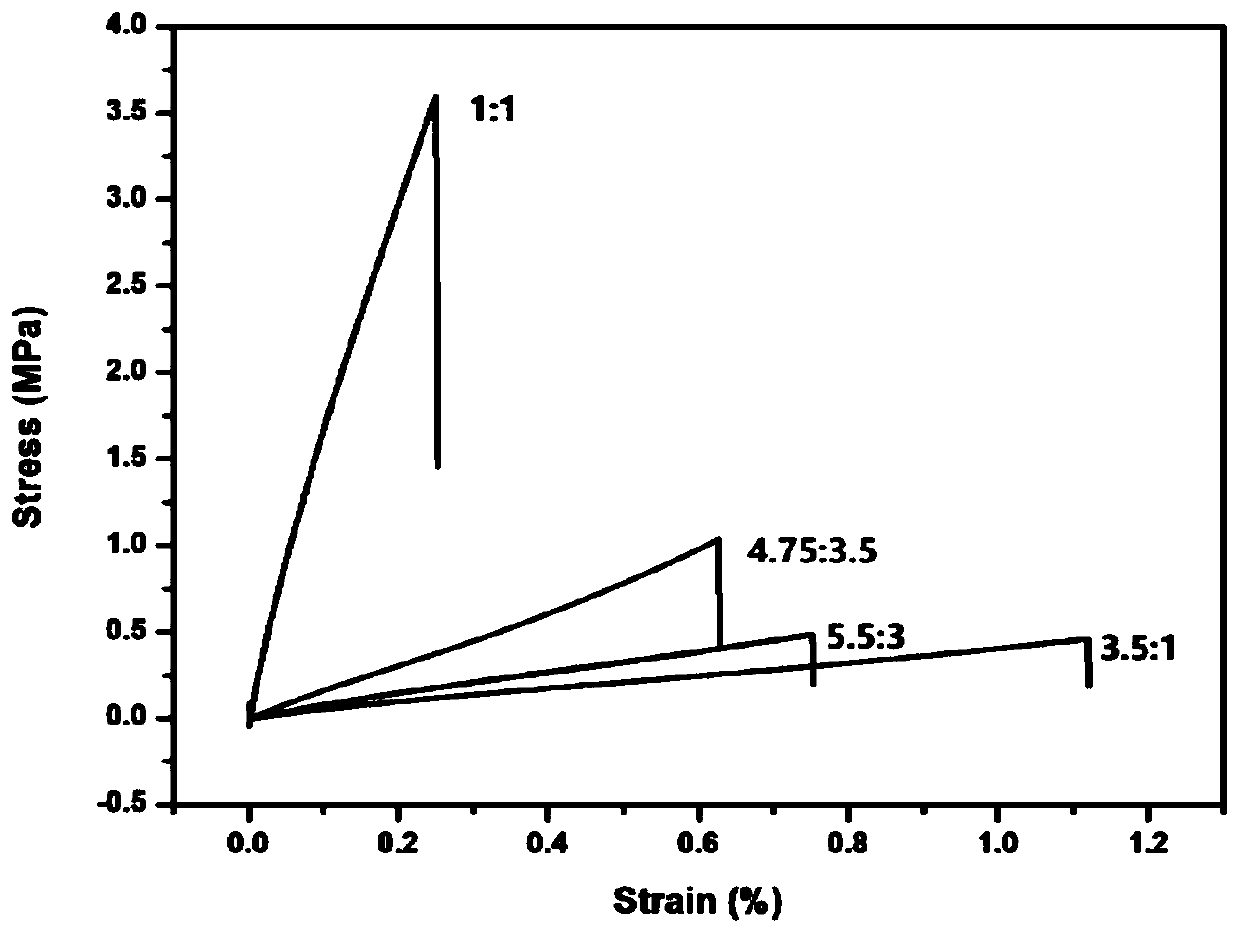
[0063] Dissolve 11gα,ω-aminopropylpolysiloxane (Mn: 2000), 2.0g glutaraldehyde (50wt%), and 0.87g tris(4-aminophenyl)amine in an appropriate amount of tetrahydrofuran (the monomer concentration is kept at about 0.1g / ml), mixed uniformly at 40°C, reacted for 2 hours, poured into a polytetrafluoroethylene mold, and dried at constant temperature until constant weight to obtain a silicone elastomer. Cut the silicone elastomer into a dumbbell shape (length×width×thickness: 50×4×0.6mm 3 ), the sample was broken with UTM2502 electronic universal testing machine at room temperature at a tensile rate of 100mm / min, the fractured surface of the sample was fully contacted, and then the sample was placed in a 100°C vacuum oven for repair for 1 hour before testing.
[0064] Figure 1-5 FT-IR images of silicone elastomers, SEM images before and after self-healing, stress-strain curves of silicone elastomers with different molar ratios of amino groups and aldehyde groups, stress-strain curve...
Embodiment 2
[0067] The procedure described in Example 1 was repeated except for 1.451 g of glyoxal (40 wt%).
Embodiment 3
[0069] The procedure described in Example 1 was repeated except for 27.5 g of α,ω-aminopropylpolysiloxane (Mn: 5000).
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com