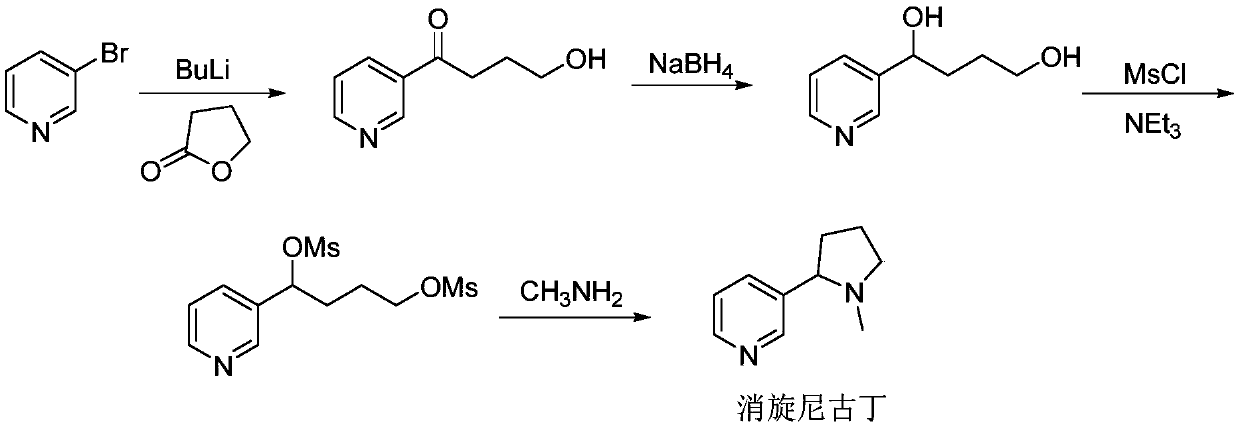
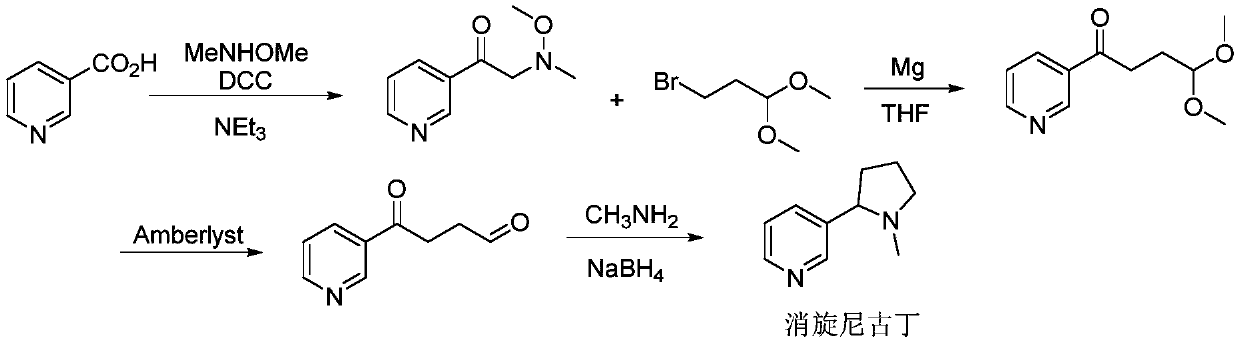
Synthesis method of (R, S-) nicotine
A synthesis method and nicotine technology, applied in the direction of organic chemistry, etc., can solve the problems of complicated separation and purification operation, difficult industrial production, expensive reagents, etc., and achieve the effect of simple operation method, easy large-scale production, and universality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]Add 2.85g of magnesium chips and 30ml of anhydrous THF to a clean three-necked flask, install the magnet, replace the condenser with a nitrogen balloon; add 2.2g of ethyl bromide initiator at one time, stir at about 25°C at room temperature, and wait for initiation (heating, Mixed); after triggering, start to drop the mixture of THF and 3-bromopyridine (the mass ratio of THF to 3-bromopyridine is 2:1, 16g 3-bromopyridine and 32g THF), keep the temperature at about 30°C Reaction; after dripping, continue to keep warm for 3 hours, and then cool to about 20°C; start to add the mixture of N-methylpyrrolidone and THF (7g N-methylpyrrolidine and 20gTHF) dropwise, and keep the temperature below 25°C After the dropwise addition, transfer to room temperature to react overnight; the next day, start to drop 6N (ie: 6mol / L) hydrochloric acid at about 25°C to quench the reaction, and adjust the pH value to between 3 and 4; then add 6N alkali The pH value of the solution was adjusted ...
Embodiment 2
[0052] Disperse the obtained 9.15g enamine intermediate in 100ml of methanol, add 1g of 5% Pd / C catalyst, place it in an autoclave, after nitrogen replacement, fill it with 1.0MPa hydrogen, and stir at room temperature overnight; after the reaction, the system The pressure was released, the reaction solution was filtered, and the filtrate was concentrated to obtain crude R,S-nicotine. After distillation, 8.3 g of a colorless and transparent pure product (68-70°C, 0.2 mmHg) was obtained. The GC purity was 99.6%, and the yield was 90%.
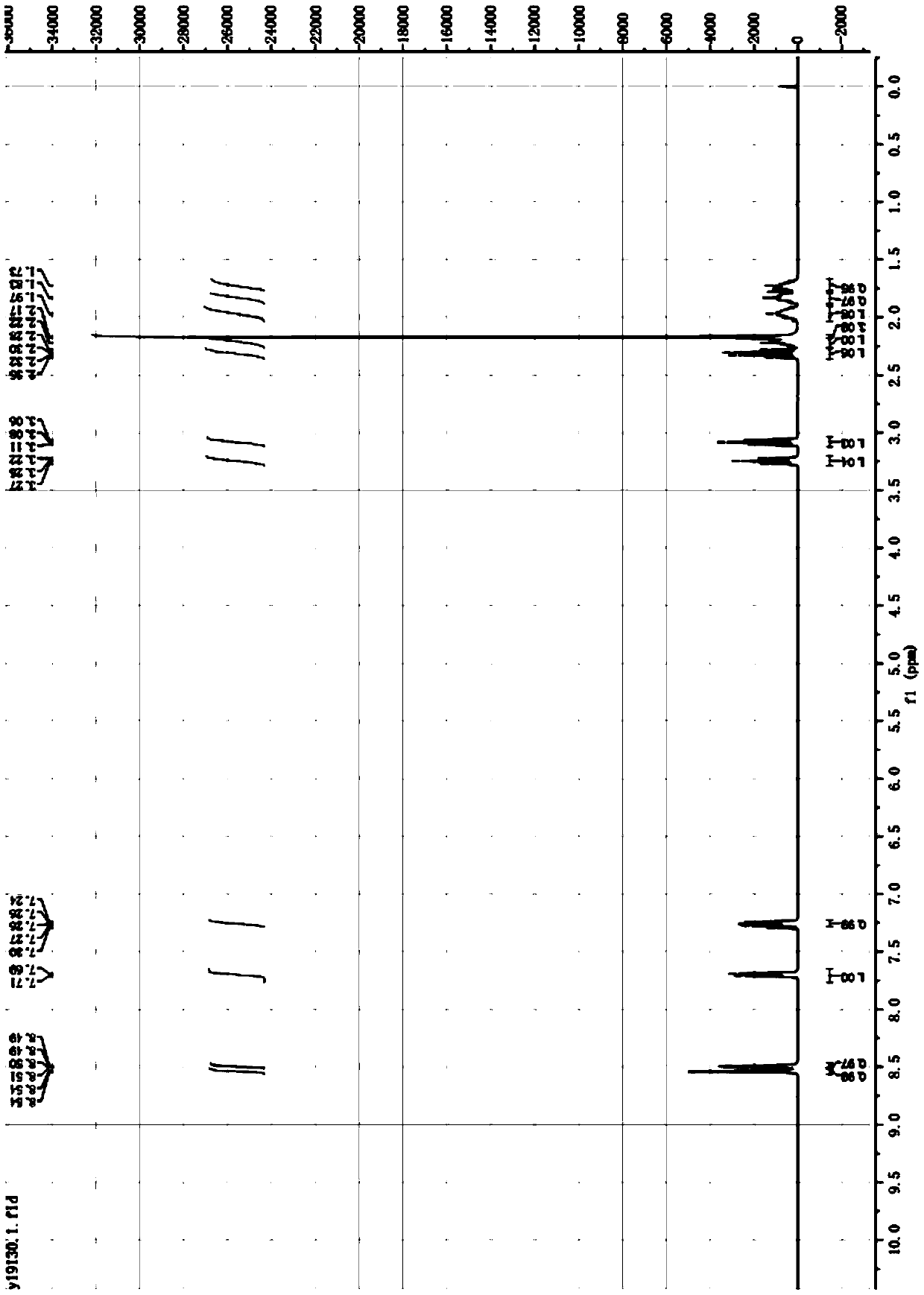
[0053] ESI-MS 163.3(M+H), l H NMR (400MHz, CDCl 3 )δ8.54(m,1H),8.50-8.49(m,1H),7.68~7.70(dt,1H),7.24~7.27(dd,1H),3.27-3.23(m,1H),3.08(t, 1H),2.28~2.34(dd,1H),2.25-2.19(m,1H),2.17(s,3H),2.03-1.91(m,1H),1.87-1.80(m,1H),1.76-1.68( m,1H). Such as figure 1 shown by figure 1 It can be seen that the R,S-nicotine prepared by the two-step method of the present application has fewer steps, simple operation, high yield, low cost, and is suitable for in...
Embodiment 3
[0055] Add 2.85g of magnesium chips and 30ml of anhydrous THF to a clean three-necked flask, install the magnet, replace the condenser with a nitrogen balloon; add 2.2g of ethyl bromide initiator at one time, stir at about 25°C at room temperature, and wait for initiation (heating, Mixed); after triggering, start to drop the mixture of THF and 3-bromopyridine (the mass ratio of THF to 3-bromopyridine is 2:1, 16g 3-bromopyridine and 32g THF), keep the temperature at about 35°C Reaction; after dripping, continue to keep warm for 3 hours, and then cool to about 20°C; start to add the mixture of N-methylpyrrolidone and THF (7g N-methylpyrrolidine and 20gTHF) dropwise, and keep the temperature below 20°C After the dropwise addition, transfer to room temperature to react overnight; the next day, start to drop 6N (ie: 6mol / L) hydrochloric acid at about 25°C to quench the reaction, and adjust the pH value to between 3 and 4; then add 6N alkali Adjust the pH value of the solution to ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com