Luminogens for biological applications
A compound and donor technology, applied in the field of red fluorescent AIE light-emitting materials, can solve the problems of weakening the performance and quenching of conventional red light-emitting bodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
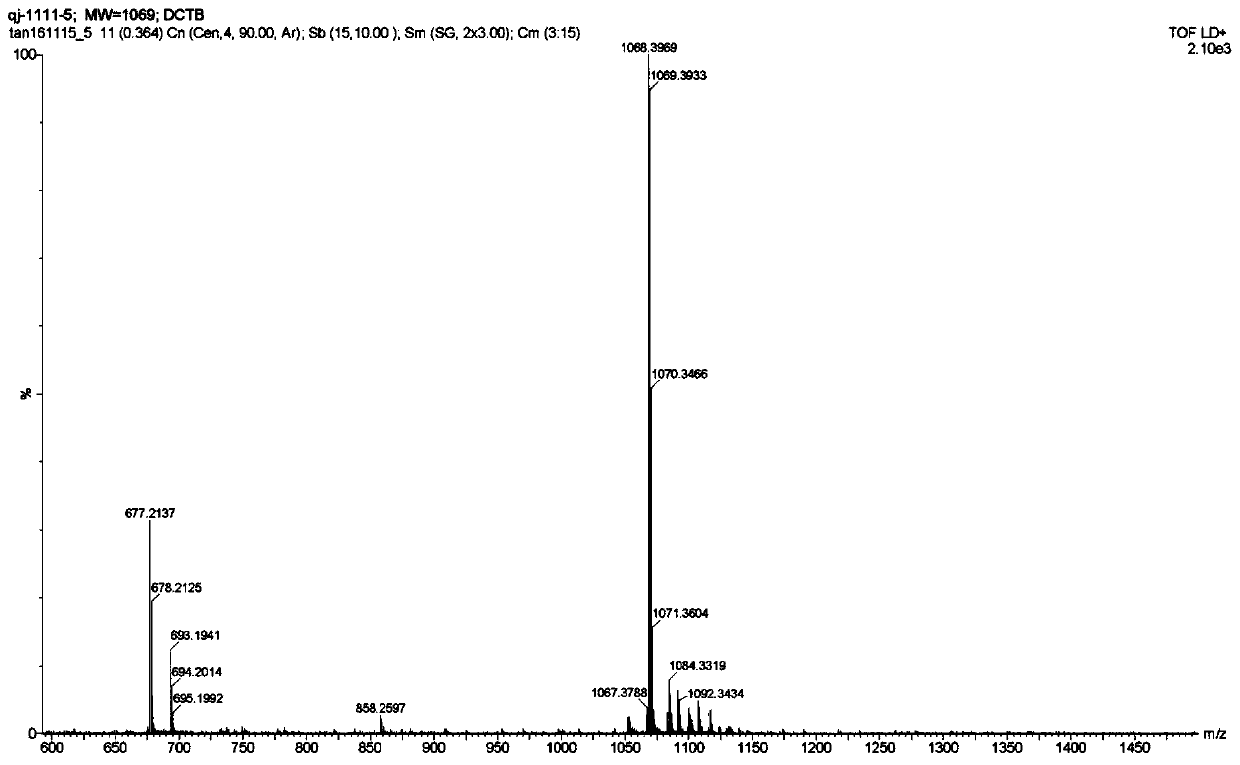
[0216] Preparation of AIE quantum dots
[0217] Typically, 1 mg AIEgen and 5 mg DSPE-PEG-Biotin are dissolved in 1 mL chloroform. Subsequently, 9 mL of Milli-Q water was added to the chloroform solution and sonicated for 10 minutes at room temperature using a microtip probe sonicator (XL2000, Misonix Incorp, USA) with an output power of 18 W. The mixture was stirred overnight under nitrogen flow until complete evaporation of chloroform. A clear solution was obtained and filtered through a 0.45 μm microfilter to form AIE quantum dots.
[0218] Cell Culture and Staining
[0219] Cell culture: in 5% CO 2 HeLa cells were cultured in MEM containing 10% FBS and antibiotics (100 units / mL penicillin and 100 μg / mL streptomycin) in a humidity incubator at 37°C. All media were supplemented with 10% heat-inactivated FBS, 100 units / mL penicillin and 100 μg / mL streptomycin. Cells were pre-cultured until confluence prior to experimentation.
Embodiment
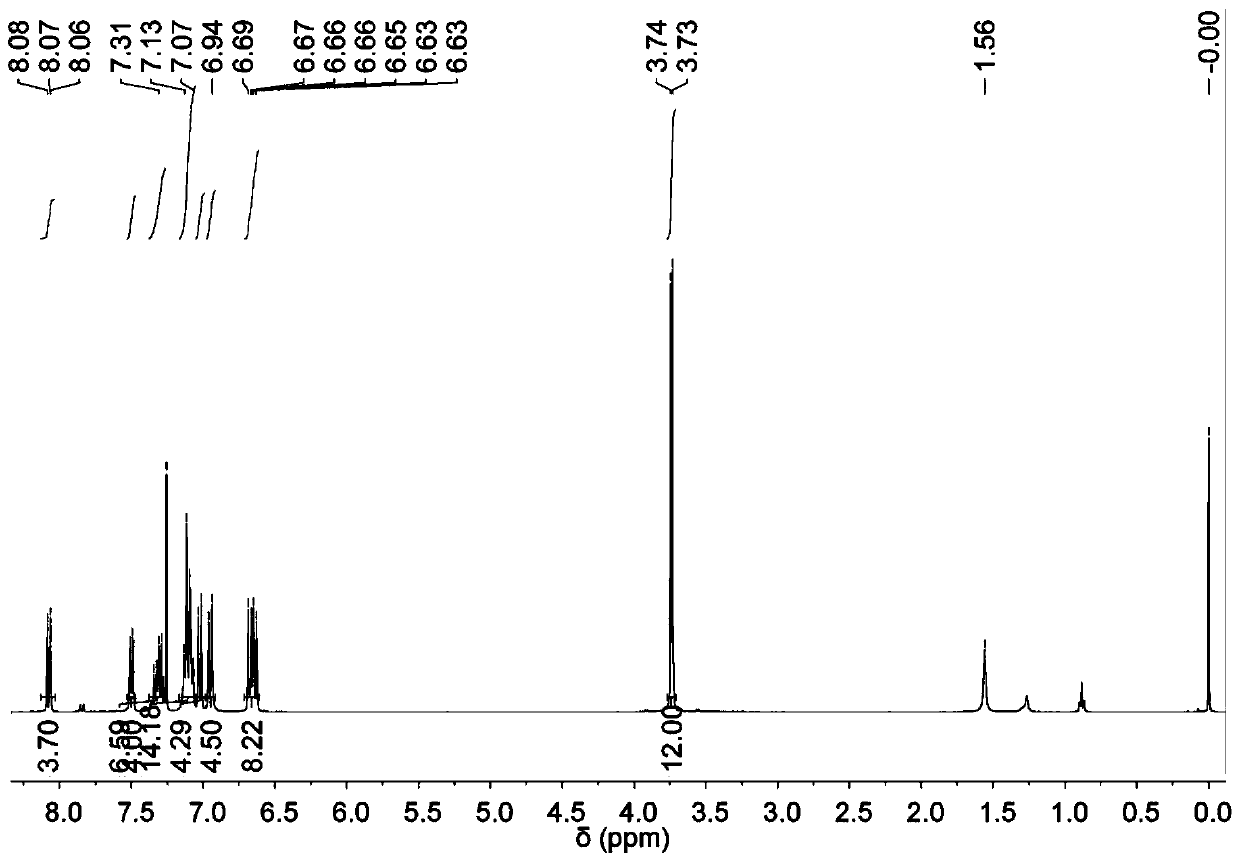
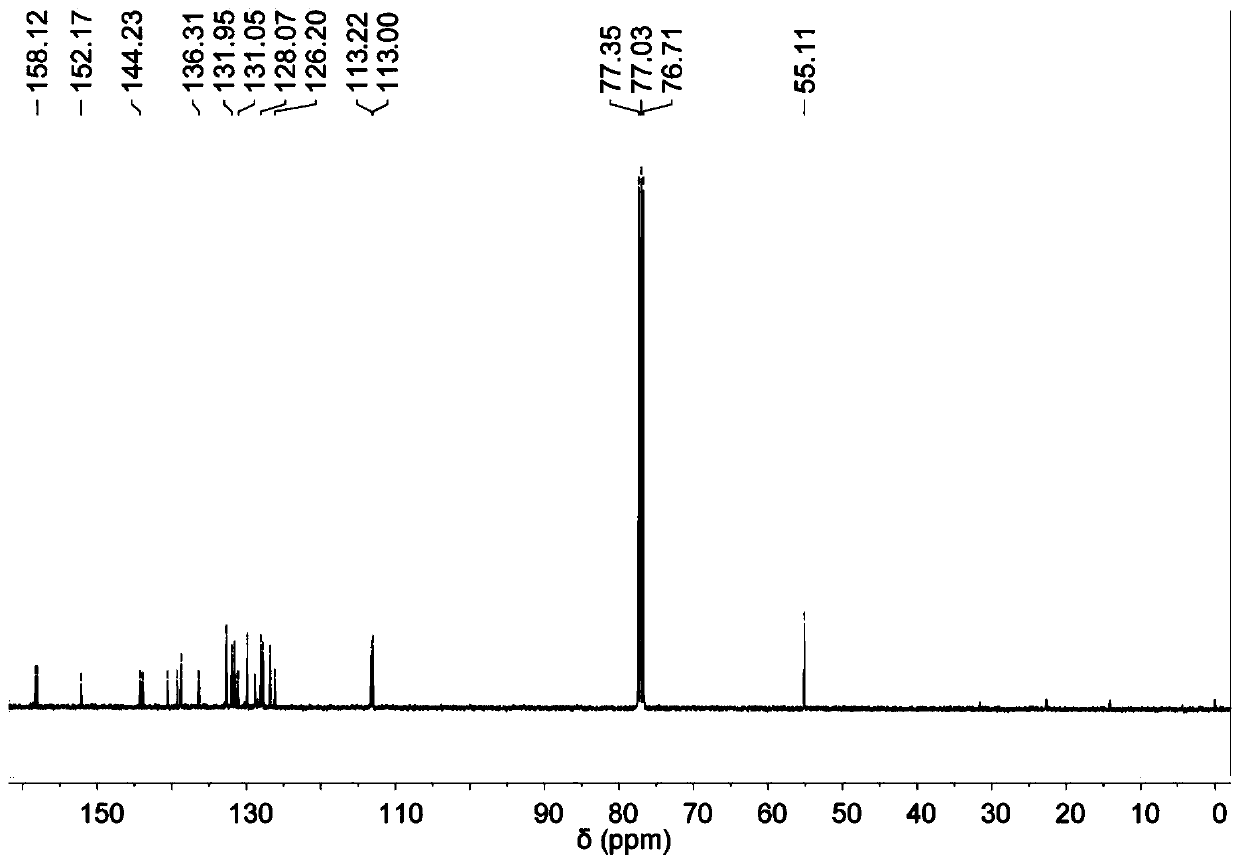
[0253] Synthesis of MTPE-TP
[0254] The synthesis of MTPE-TP is shown in Scheme 1 below.
[0255] Scheme 1: Synthetic route of MTPE-TP
[0256]
[0257] 4,4'-Dimethoxybenzophenone (20 mmol, 4.84 g), 4-bromobenzophenone (24 mmol, 6.26 g) and zinc powder (80 mmol, 5.2 g) were added to a 500 mL round bottom flask middle. The flask was then evacuated under vacuum and flushed three times with dry nitrogen. After adding 250 mL of anhydrous THF, the mixture was cooled to −10 °C and TiCl was added dropwise4 (7.7 mL, 70 mmol) was stirred for 30 minutes. The mixture was heated to reflux and stirred for 12 hours. After cooling to room temperature, the reaction was quenched with 1M HCl solution, and the mixture was extracted three times with dichloromethane. Combine the organic phases with MgSO 4 Dry, and evaporate the solvent under reduced pressure. The residue was purified by silica gel column chromatography using dichloromethane / hexane (v / v 1:5) as eluent to give 4,4'-(2-(4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrodynamic diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com