PhSGBL polymer, and preparation method and application thereof
A bicyclic lactone and polymer technology, which is applied in the field of bicyclic lactone polymer and its preparation and application, can solve the problems of small polymer molecular weight, poor impact resistance, and reduced hydrophilicity, and achieve narrow molecular weight distribution, Broaden application scenarios and controllable molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The preparation of embodiment 1PPhSGBL
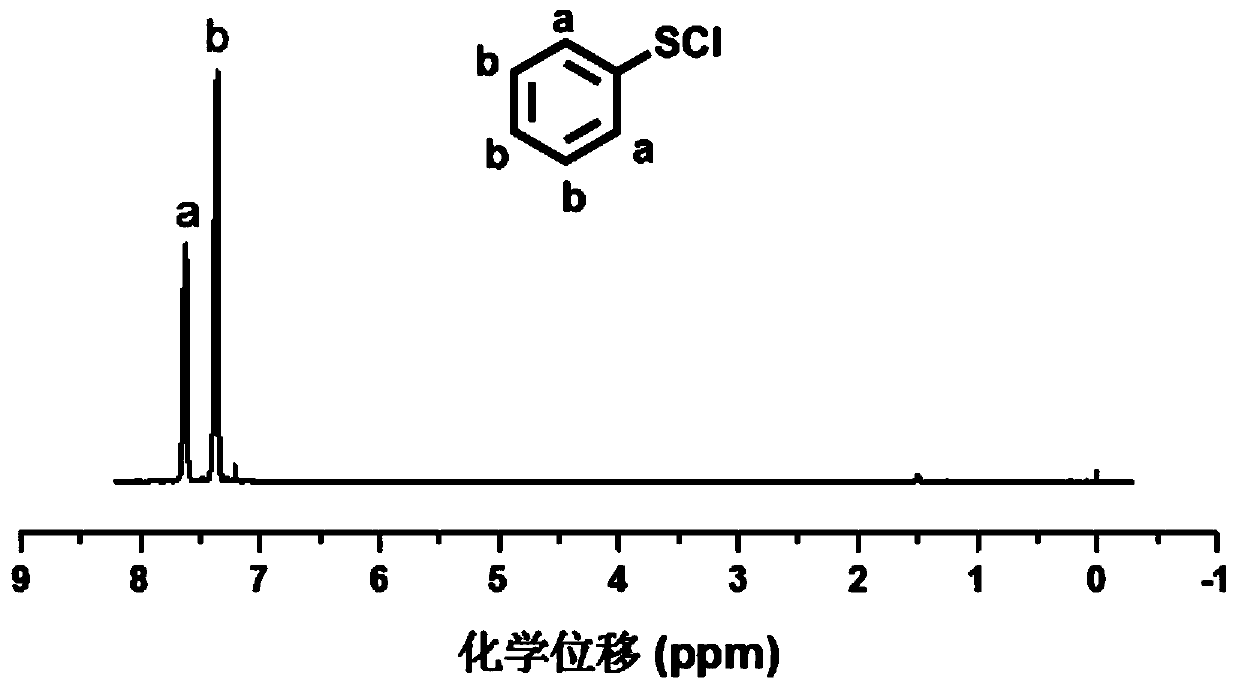
[0069] 1) Synthesis of phenylsulfur chloride (PhSCl)
[0070] At 0°C, diphenyl disulfide (10 g, 45.8 mmol) and triethylamine (0.65 ml, 4.6 mmol) were dissolved in 75 ml of benzene and stirred under argon atmosphere for 15 min. Then, sulfonyl chloride (14.73g, 110mmol) was dissolved in 110mol of dichloromethane, and slowly added dropwise to the above solution. After the dropwise addition, the mixture was continuously stirred for 1h and the reaction was stopped. The reaction solution was concentrated by rotary evaporation to remove the solvent, and then subjected to vacuum distillation (0.75 torr, 50° C.) to obtain phenylsulfur chloride as an orange liquid (10.7 g, yield: 81.7%). Above synthetic route is as follows:
[0071]
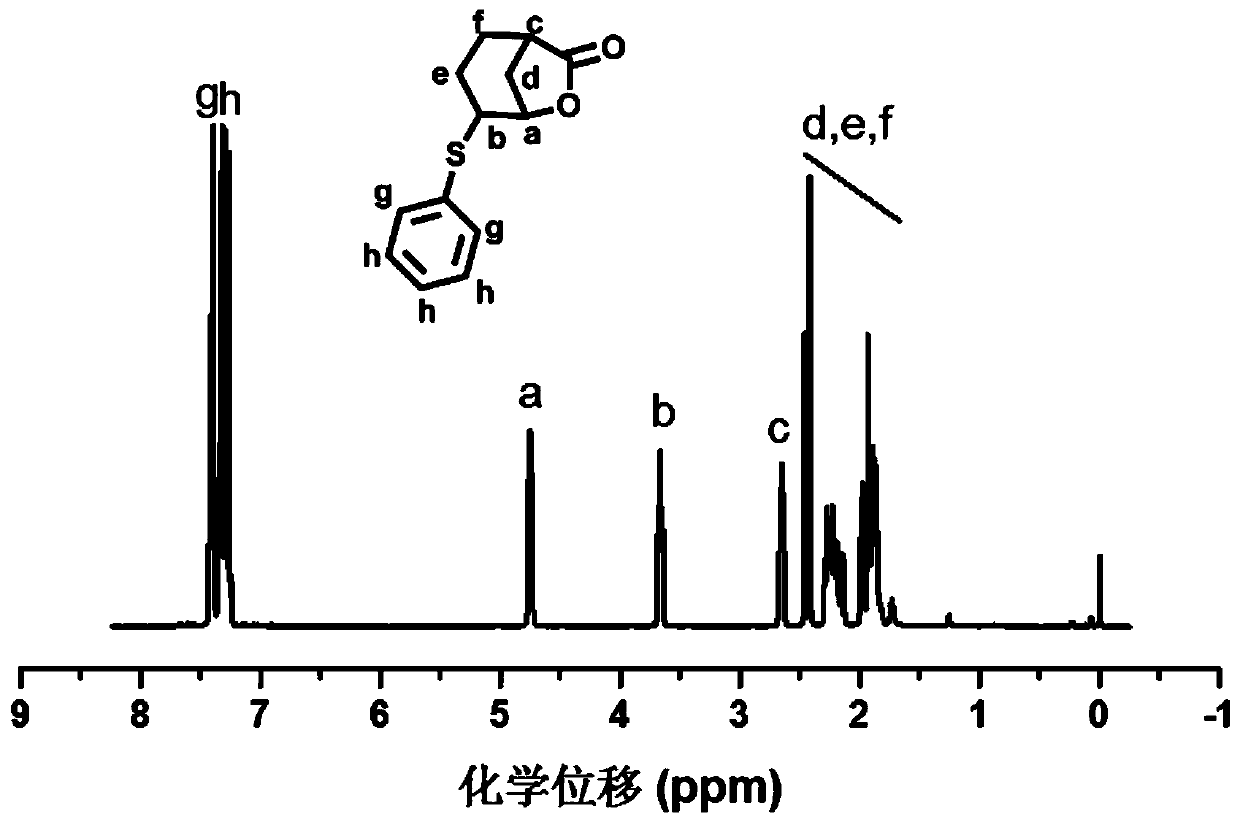
[0072] 2) Synthesis of lactone monomer PhSGBL
[0073] 3-cyclohexene-1-carboxylic acid (7g, 55mmol) and triethylamine (7.7ml, 55mmol) were dissolved in 50ml of dichloromethane at room temperature, stirr...
Embodiment 2
[0081] The random copolymer synthesis of embodiment 2PPhSGBL and CL
[0082] In the glove box, according to the scale [CL] 0 / [PhSGBL] 0 =0 / 100, 20 / 80, 40 / 60, 60 / 40, 80 / 20, 100 / 0 (when the endpoint value is 0, it means that the reaction monomer is not contained) the monomer PhSGBL (0.4g, 1.71mmol), ε-caprolactone (the amount added is calculated according to the proportion), organic base 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) (9.52mg, 0.0684mmol), initiator ethylenedi Alcohol (EG) (1.91ml, 0.0342mmol) and 1ml solvent dichloromethane (DCM) were added to the reaction vessel, the reaction temperature was 30°C, and the reaction was stopped after stirring for 13-15h.
[0083] After the reaction, it was dissolved with 2ml of tetrahydrofuran (THF), then dropped into glacial ether to settle, obtained by suction filtration, and dried in a vacuum oven at 40°C for 12 hours to obtain random copolyesters with different proportions of PhSGBL and CL. Above synthetic route is as follows:...
Embodiment 3
[0089] Embodiment 3 contains the synthesis of sulfoxide or sulfone polymer
[0090] Polyester PPhSGBL (100mg, M n =16000, ), 20ml of solvent chloroform (DCM) were added to the reaction vessel, the reaction temperature was set at 25°C, and the oxidant m-chloroperoxybenzoic acid (m-CPBA) was gradually added to it, stirring for 10-15min after each addition, and at the same time according to In the above method, multiple groups of parallel experiments were performed, and different amounts of oxidants were added to each group, and then a small amount of reaction solution was taken for FT-IR testing, as shown in Figure 11 as shown. Figure 11 Among them, the molar ratios of oxidant and PPhSGBL corresponding to the top-down curve are 0:1, 0.27:1, 0.54:1, 0.81:1, 1.08:1, 1.35:1, 1.62:1, 1.89:1, 2.16:1, 2.43:1, 2.70:1, in the picture, 1041.5cm -1 The peak at is the characteristic peak of S=O bond, 1303.8cm -1 The peak at is the characteristic peak of the O=S=O bond, and the resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com