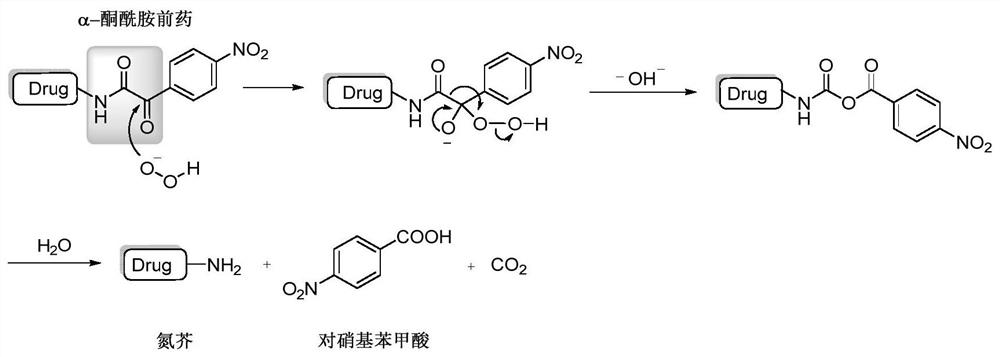
A hydrogen peroxide-responsive nitrogen mustard antitumor prodrug and its preparation method
A nitrogen mustard and preparation technology, applied in the field of nitrogen mustard anti-tumor prodrugs and their preparation, can solve the problems of poor tumor cell selectivity, short half-life, poor selectivity, etc., achieve rich structural types, reduce toxic side effects, and improve selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the synthesis of compound C
[0074] The first step: the synthesis of p-nitrophenylglyoxylic acid (intermediate 1)
[0075]
[0076] Concrete operation: p-Nitroacetophenone (1g) is dissolved in pyridine (10mL), adds SeO 2 (1g), the mixture was passed through nitrogen protection, and stirred at 90°C for 4h, wherein p-nitroacetophenone and SeO 2 The molar ratio is 1:1.5, cooling and filtering after the reaction is completed, the filter cake is washed 3 times with ethyl acetate, and the organic layer is collected and washed with 2M HCl, and finally the organic layer is combined, dried with anhydrous sodium sulfate, and the solvent is removed by distillation under reduced pressure. The yellow intermediate 1 was eluted by column chromatography.
[0077]The second step: the synthesis of N-(4-nitrophenyl)diethanolamine (intermediate 4)
[0078]
[0079] Specific operation: Dissolve p-nitrofluorobenzene (1mL) in dimethyl sulfoxide (DMSO), then add diethan...
Embodiment 2
[0090] Embodiment 2: the synthesis of compound A
[0091]
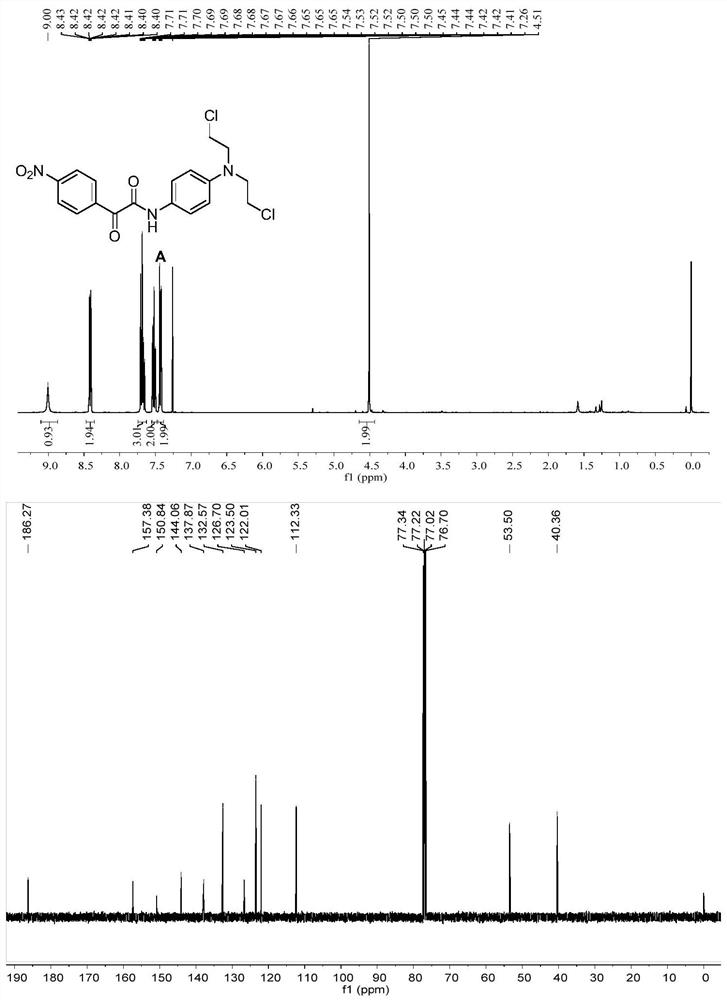
[0092] Specific operation: Dissolve compound C (100mg) in DMF (3mL), add halide LiCl (40.07mg), stir at 60°C for 3h, after the reaction, wash with water and saturated brine in sequence, add ethyl acetate for extraction, The organic layer was collected, dried by adding anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and eluted by column chromatography to obtain a dark brown solid A. 1 H NMR (400MHz, Chloroform-d) δ8.83(s,1H,NH),8.64–8.56(m,2H,Ar-H),8.38–8.29(m,2H,Ar-H),7.63–7.56( m,2H,Ar-H),6.78–6.68(m,2H,Ar-H),3.76(m,2H,NCH 2 ),3.65(m,2H,ClCH 2 ). 13 C NMR (101MHz, Chloroform-d) δ186.27, 157.38, 150.84, 144.06, 137.87, 132.57, 126.70, 123.50, 122.01, 112.33, 53.50, 40.36. Mass spectrometry (ESI-MS, m / z): Calcd for [M+ H] + ,409.06; found 410.061.m.p:141–143℃.
Embodiment 3
[0093] Embodiment 3: the synthesis of compound B
[0094]
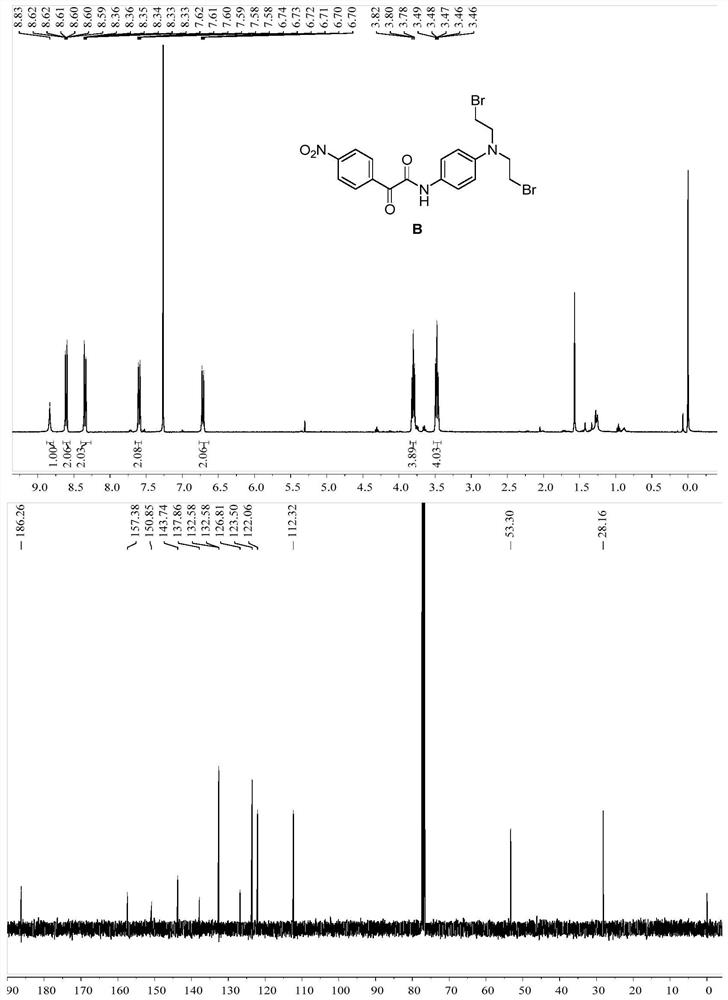
[0095] Specific operation: Dissolve compound C (100mg) in DMF (3mL), add halide NaBr (97mg), stir at 60°C for 3h, after the reaction, wash with water and saturated brine, add ethyl acetate for extraction, collect The organic layer was dried by adding anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and column chromatography was eluted to obtain dark brown solid B. 1 H NMR (400MHz, Chloroform-d) δ8.83(s,1H,NH),8.65–8.56(m,2H,Ar-H),8.40–8.29(m,2H,Ar-H),7.65–7.54( m,2H,Ar-H),6.77–6.65(m,2H,Ar-H),3.80(t,J=7.5Hz,2H,NCH 2 ), 3.48 (dd, J=8.1, 6.9Hz, 2H, BrCH 2 ). 13 C NMR (101MHz, Chloroform-d) δ186.26, 157.38, 150.85, 143.74, 137.86, 132.58, 126.81, 123.50, 122.06, 112.32, 53.30, 28.16. Mass spectrometry (ESI-MS, m / z): Calcdfor[M+H ] + ,496.96; found 497.963.m.p:95–96℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com