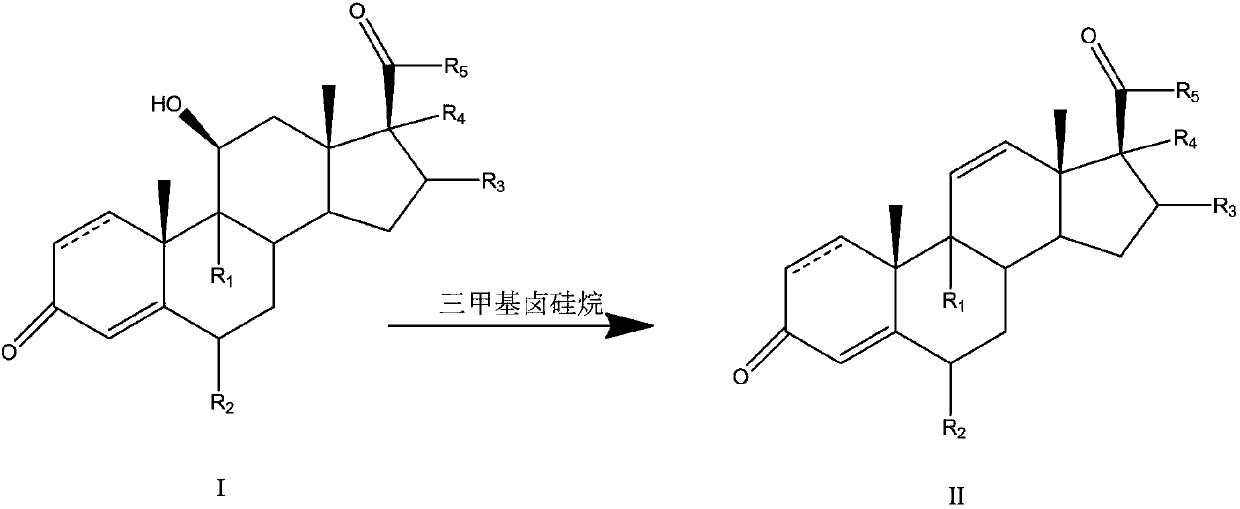
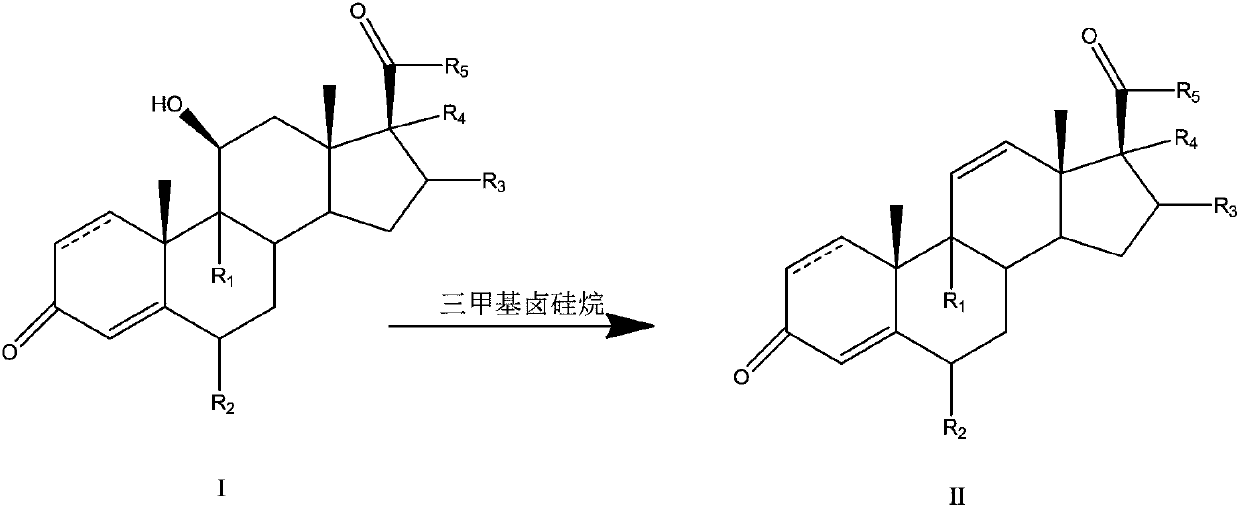
Preparation method of 11-ene steroid compound
A technology of compounds and enosteroids, which is applied in the field of preparation of 11 enosteroids, can solve the problems of unsuitability for industrial production, high price, and high price of fluorine reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
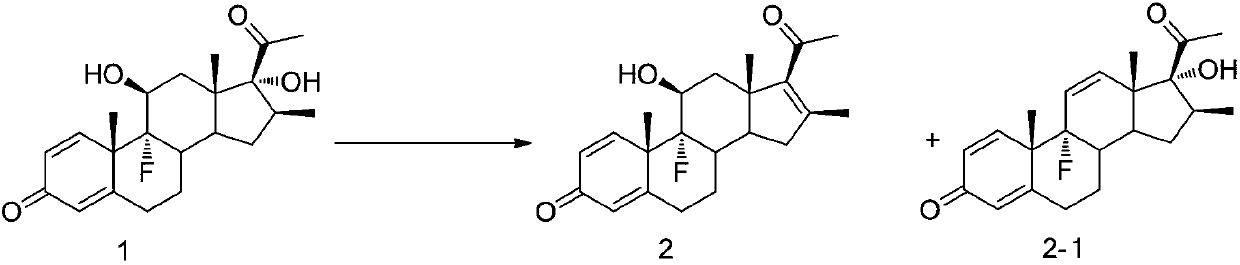
Embodiment 1
[0044]
Embodiment 1-1
[0046] Compound 1 (5g, 13.5mmol) was dissolved in acetonitrile (100ml), trimethyl iodosilane (3.8ml, 27.0mmol) was added, and the reaction was stirred at 50°C. The reaction progress was monitored by TLC until the reaction was complete, and Na 2 S 2 o 3 aq (300ml, 10% w / v) to terminate the reaction, add 200ml ethyl acetate to extract and separate liquid, after vacuum distillation, obtain white solid 2-1 (4.4g) by recrystallization from ethyl acetate / petroleum ether, purity 99.2% , 92% molar yield. A small amount of compound 2 was detected in the mother liquor.
Embodiment 1-2
[0048] Compound 1 (5g, 13.5mmol) was dissolved in acetonitrile (100ml), trimethyl iodosilane (5.8ml, 40.5mmol) was added, the reaction was stirred at 50°C, the reaction progress was monitored by TLC, until the reaction was complete, Na 2 S 2 o 3aq (300ml, 10% w / v) terminated the reaction, separated the liquids, extracted the liquids with 200ml ethyl acetate, combined the organic phases for vacuum distillation, and obtained white solid 2 (4.4g) by recrystallization from ethyl acetate / petroleum ether. The purity is 98.5%, and the molar yield is 92%. It was detected that a small amount of compound 2-1 was formed in the mother liquor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com