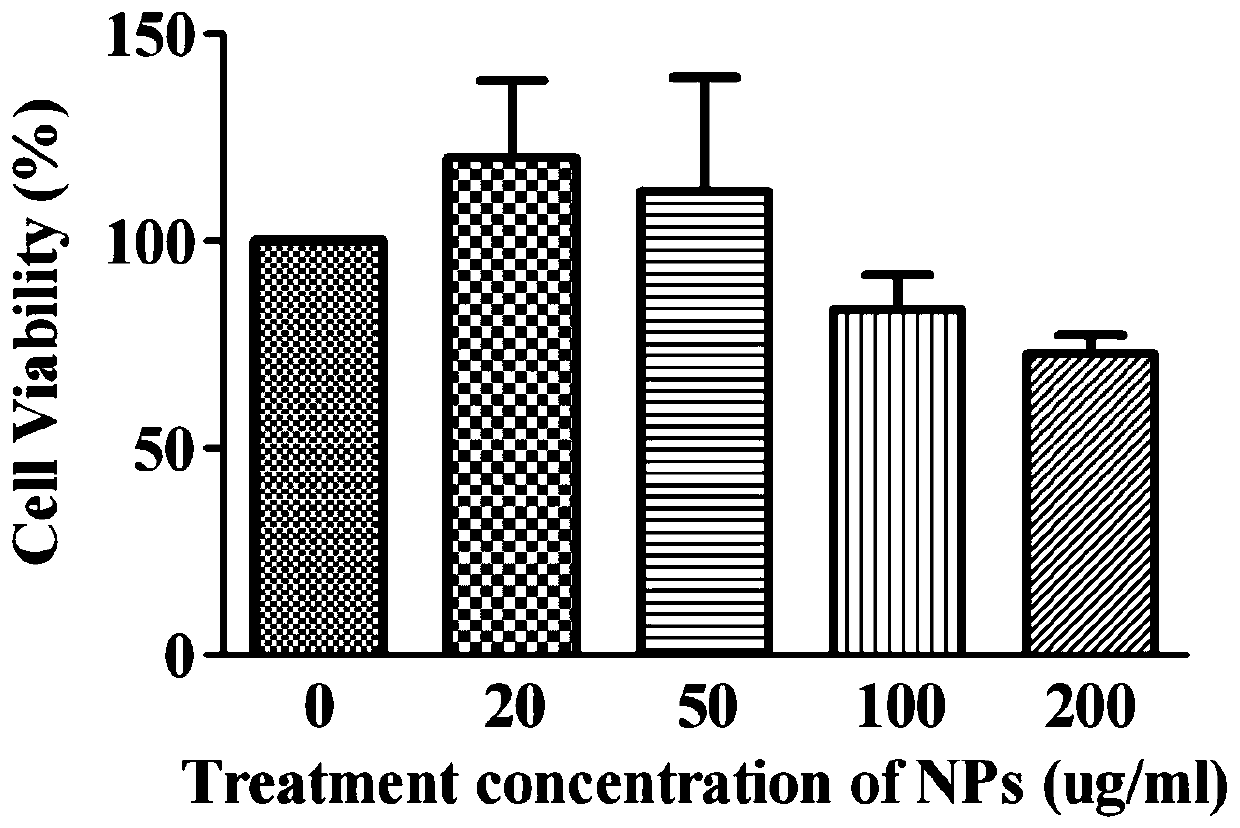
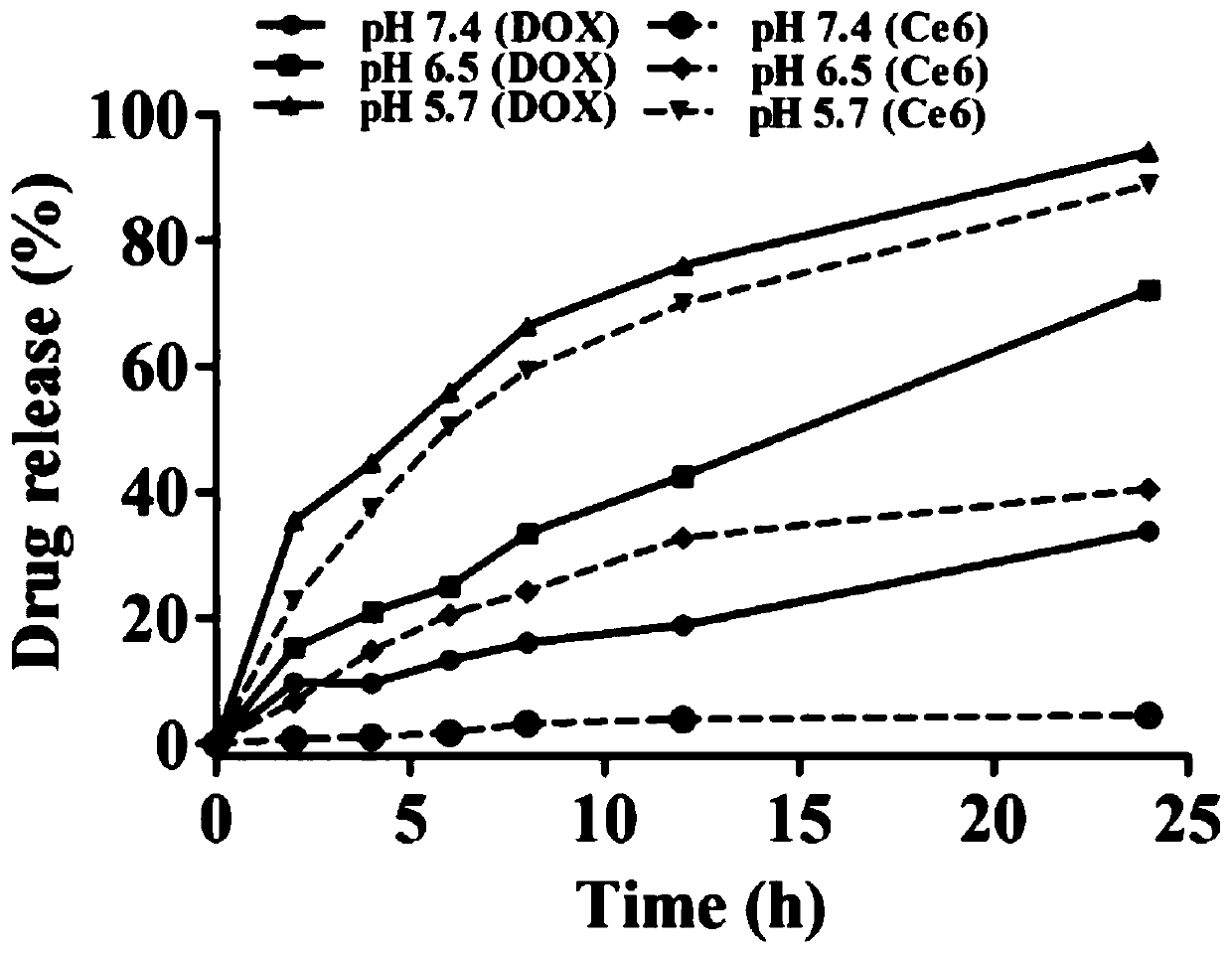
Silk fibroin/manganese dioxide composite microsphere drug carrier with core-shell structure and preparation method thereof
A technology of silk fibroin and manganese dioxide, which is applied in the direction of non-active components of polymer compounds, drug combinations, and pharmaceutical formulations, can solve problems such as harsh reaction conditions, unstable properties of nanoparticles, and complex synthesis techniques of nanoparticles, and achieve The effect of simple process, excellent singlet oxygen generation ability, and controllable drug release ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation method of the silk fibroin / manganese dioxide composite microsphere drug carrier of the core-shell structure in this embodiment comprises the following steps in sequence:
[0041] (1) Take an appropriate amount of silkworm cocoon shells, wash and dry them, and then use the existing method to perform degumming treatment. Subsequently, the degummed silk fibers are sequentially subjected to the existing steps of dissolving, dialysis, and concentration to obtain silkworm silk fibroin aqueous solution;
[0042] (2) Adjust the silk fibroin concentration in step (1) to 2mg / mL (the solvent is water, the same as other embodiments), and take 10mL and place it in a reaction vessel. Slowly add 1mL of isopropanol dropwise to the silk protein solution, and keep stirring for 30min to make it evenly mixed.
[0043] (3) The mixed solution in step (2) was placed in a -80°C refrigerator, frozen for 12 hours, and then allowed to thaw naturally at room temperature to obtain ...
Embodiment 2
[0049] The preparation method of the silk fibroin / manganese dioxide composite microsphere drug carrier of the core-shell structure in this embodiment comprises the following steps in sequence:
[0050] (1) Take an appropriate amount of silkworm cocoon shells, wash and dry them, and then perform degumming treatment. Subsequently, the degummed silk fibers are sequentially subjected to steps such as dissolving, dialysis, and concentration to obtain an aqueous solution of silkworm silk fibroin;
[0051] (2) Adjust the silk fibroin concentration in step (1) to 2%, take 5 mL and place it in a reaction vessel, add 2 mL of absolute ethanol and stir evenly.
[0052] (3) The mixed solution in step (2) was placed in a -20°C refrigerator, frozen for 24 hours, and thawed naturally at room temperature to obtain a silk protein suspension. The silk protein suspension was centrifuged, washed and sonicated to obtain silk protein microspheres with a diameter in the range of 200-500 nm, and resu...
Embodiment 3
[0055] Embodiment 3 (in situ drug loading)
[0056] The preparation method of the silk fibroin / manganese dioxide composite microsphere drug carrier of the core-shell structure in this embodiment comprises the following steps in sequence:
[0057] (1) Take an appropriate amount of silkworm cocoon shells, wash and dry them, and then perform degumming treatment. Subsequently, the degummed silk fibers are sequentially subjected to steps such as dissolving, dialysis, and concentration to obtain an aqueous solution of silkworm silk fibroin;
[0058] (2) Adjust the concentration of silk fibroin in step (1) to 2mg / mL, take 10mL and place it in a reaction vessel, add 2mg of doxorubicin hydrochloride (DOX) to the silk fibroin solution, and ultrasonically disperse it to mix with silk Vegetarian protein was mixed evenly, and then 1 mL of isopropanol was slowly added dropwise to the reaction solution, and stirring was continued for 30 min to make it evenly mixed.
[0059] (3) The mixed s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com