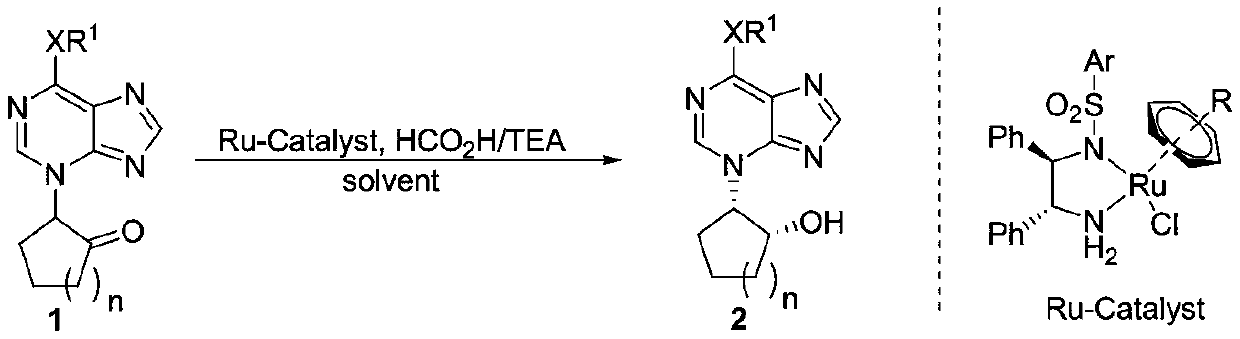
Method used for synthesis of chiral cis-carbocycle N3-purine nucleoside
A purine nucleoside and chirality technology, which is applied in the field of synthesizing chiral cis-carbocyclic N3-purine nucleosides, can solve the problems of cumbersome process and high cost, and achieves high reaction yield, high-efficiency synthesis method, and easy-to-obtain reaction raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023]
[0024]
[0025] [a] Unless otherwise specified, the reaction steps are as follows: 1a (0.1mmol), 0.5-2% catalyst, 1mL solvent, HCO 2 H / TEA (molar ratio 1:1) was reacted at 27°C for 1 day [b] dr>20:1, judged by NMR detection of crude product [c] separation yield [d] ee value was analyzed by chiral column [ e] HCO 2 H / TEA=2.5:1[f]HCO 2 H / TEA=0.2:1.
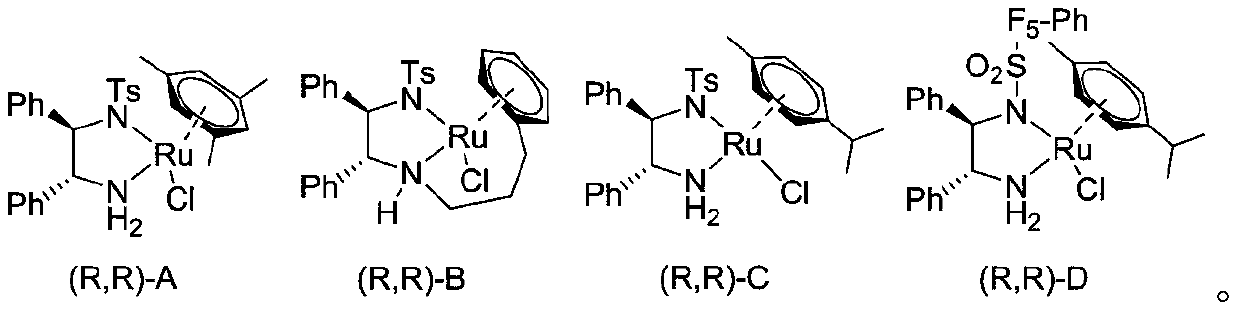
[0026] In the screening process of reaction conditions, the influence of metal catalysts on the reaction was firstly investigated (markers 1-4). At the same time, by comparing the effects of different ligands on the reaction and considering the price factor, the catalyst (R,R)-D was finally determined to be the best catalyst. Afterwards the best solvent dioxane, HCO was selected 2 H / TEA=1:1.
[0027] The investigation of reaction condition: (taking label 13 as example)
[0028] In a 10mL reaction flask, add α-(6-diethylamino-3H-purinyl)cyclopentanone 1a (27.5mg, 0.1mmol), catalyst (R,R)-D (0.7mg, 1mo...
Embodiment 2
[0030]
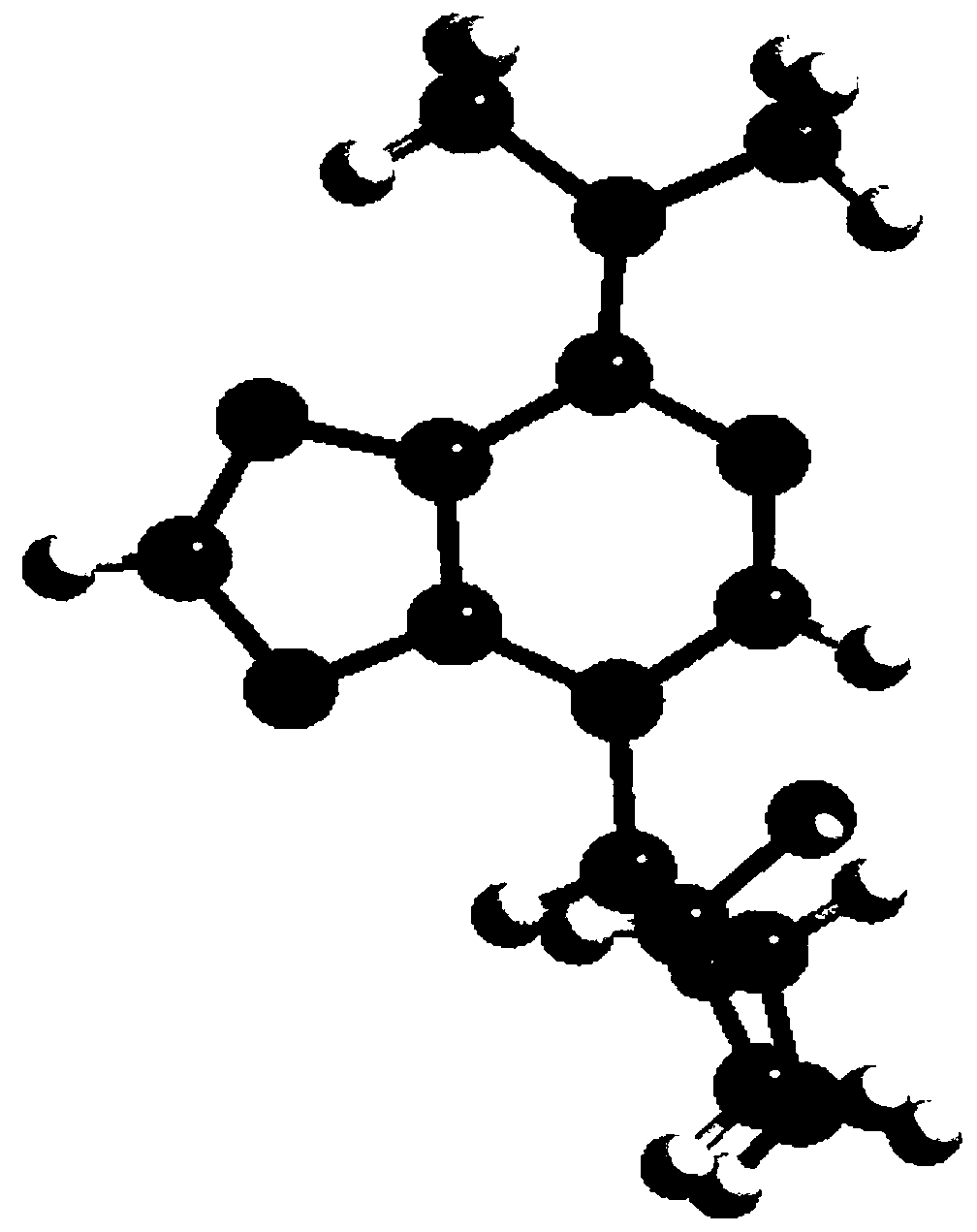
[0031] In a 10mL reaction flask, add α-(6-dimethylamino-3H-purinyl)cyclopentanone 1b (24.5mg, 0.1mmol), catalyst (R,R)-D (0.7mg, 1mol%) and 1 mL of dioxane. Then add HCO 2 H / TEA=1:1 mixed solvent 70 μL, put the reaction tube at 27°C for 24 hours. Track the reaction with TLC. After the reaction is terminated, the organic phase is concentrated in vacuo, and column chromatography obtains a white solid 2b (single crystal X-ray diffraction is figure 1 ), yield 95%, 99.9%ee, dr>20:1, melting point: 230.5-234.9°C. [α] D 20 =-72.89 (c=0.584, CH 2 Cl 2 ); 1 H NMR (600MHz, CDCl 3 )δ8.14(s,1H),7.59(s,1H),4.86(dt,J=11.4,5.4Hz,1H),4.70(d,J=5.4Hz,1H),3.54(s,3H), 3.26(s,3H),2.39-2.31(m,1H),2.27-2.21(m,1H),2.09-2.02(mm,2H),1.95-1.90(m,1H),1.72-1.64(m,1H ); 13 C NMR (150MHz, CDCl 3 )δ152.5, 152.1, 150.4, 140.3, 119.7, 70.1, 63.7, 39.7, 38.0, 32.8, 26.6, 20.5; HRMS (ESI-TOF): exact mass calcd for C 12 h 18 N 5 O(M+H) + requires m / z 248.1506, found m / z 248.1507.
Embodiment 3
[0033]
[0034] In a 10mL reaction flask, add α-(6-ethoxy-3H-purinyl)cyclopentanone 1c (24.8mg, 0.1mmol), catalyst (R,R)-D (0.7mg, 1mol%) and 1mL Dioxane. Then add HCO 2H / TEA=1:1 mixed solvent 70 μL, put the reaction tube at 27°C for 24 hours. The reaction was tracked by TLC. After the reaction was terminated, the organic phase was concentrated in vacuo, and column chromatography obtained a white solid 2c with a yield of 94%, 95% ee, dr>20:1, melting point: 215.0-218.9°C. [α] D 20 =-133.66 (c=0.153, CH 2 Cl 2 ); 1 H NMR (600MHz, CD 3 OD):8.75(s,1H),8.08(s,1H),5.24-5.20(m,1H),4.79-4.75(m,2H),4.47-4.46(m,1H),2.56-2.49(m, 1H),2.30-2.25(m,1H),2.23-2.17(m,1H),2.15-2.09(m,1H),1.93-1.80(m,2H),1.52(t,J=7.2Hz,3H) ; 13 C NMR (150MHz, CD 3 OD):161.2,155.7,155.4,143.9,123.8,71.5,65.7,65.6,33.7,27.5,21.1,14.9; HRMS(ESI-TOF):exact mass calcd for C 12 h 17 N 4 o 2 (M+H) + requires m / z 249.1346, found m / z 249.1345.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com