A kind of FEBC amorphous alloy Fenton-like method for catalytic degradation of methylene blue sewage
An amorphous alloy, methylene blue technology, applied in the field of metal materials, can solve the problem of secondary pollution of the solution, and achieve the effect of easy operation, low degradation time, and realization of degradation and removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
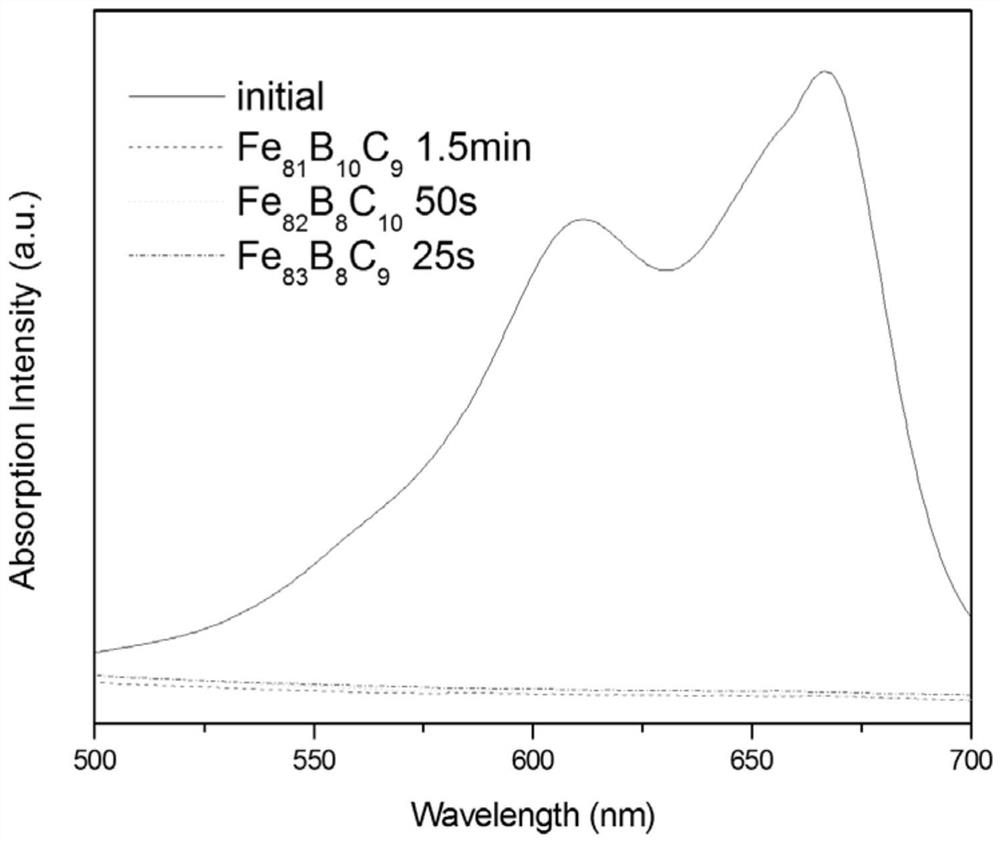
[0035] At 25°C, take 100ml of 20mg / L methylene blue solution and adjust the pH value to 5 with dilute sulfuric acid. Aspirate 0.5 g of Fe with a magnetic stirrer rotor 81 B 10 C 9 For amorphous flakes, use a magnetic stirrer to stir the solution, add 0.5 ml of 30% mass fraction hydrogen peroxide, carry out magnetic stirring for 1.5 min, and use an ultraviolet spectrophotometer for photometric analysis.
[0036] Analysis results such as figure 1 As shown, when the reaction was carried out for 1.5 min, the characteristic peak of methylene blue had basically disappeared, indicating that the concentration of methylene blue was already very low at this time, and the dye had been basically removed.
Embodiment 2
[0038] At 25°C, take 100ml of 20mg / L methylene blue solution and adjust the pH value to 4 with dilute sulfuric acid. Aspirate 0.9 g of Fe with a magnetic stirrer rotor 82 B 8 C 10 For amorphous flakes, use a magnetic stirrer to stir the solution, add 0.9 ml of 30% mass fraction hydrogen peroxide, carry out magnetic stirring for 50 s, and use an ultraviolet spectrophotometer for photometric analysis.
[0039] Analysis results such as figure 1 As shown, when the reaction was carried out for 50 s, the characteristic peak of methylene blue had basically disappeared, indicating that the concentration of methylene blue was already very low at this time, and the dye had been basically removed.
Embodiment 3
[0041] At 25°C, take 100ml of 20mg / L methylene blue solution and adjust the pH to 3 with dilute sulfuric acid. Aspirate 1 g of Fe with a magnetic stirrer rotor 83 B 8 C 9 For amorphous flakes, use a magnetic stirrer to stir the solution, add 1 ml of 30% mass fraction hydrogen peroxide, carry out magnetic stirring for 25 s, and use an ultraviolet spectrophotometer for photometric analysis.
[0042] Analysis results such as figure 1 As shown, when the reaction was carried out for 25s, the characteristic peak of methylene blue had basically disappeared, indicating that the concentration of methylene blue was already very low at this time, and the dye had been basically removed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com