Olmesartan medoxomil amlodipine tablet and preparation method thereof
A technology of amlodipine ester amlodipine tablets and olmesartan medoxomil, applied in the field of medicine, can solve the problems of affecting drug dissolution and stability, reducing drug bioavailability, increasing drug cost, etc., achieving good clinical application prospects and compatibility The effect of reducing problems and dispersing quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of olmesartan medoxomil amlodipine tablet, its prescription contains the following components by weight: 40 parts of olmesartan medoxomil, 10 parts of amlodipine besylate, 60 parts of silicified microcrystalline cellulose, 30 parts of pregelatinized starch 4 parts of croscarmellose sodium, 30 parts of calcium hydrogen phosphate, 4 parts of sodium starch glycolate, 1 part of magnesium stearate, 0.5 parts of titanium dioxide, 0.5 parts of talc, 4 parts of coating premix .
[0033] The preparation method of described olmesartan medoxomil amlodipine sheet, comprises the following steps:
[0034] (1) Get olmesartan medoxomil and amlodipine besylate and pulverize through a 100-mesh sieve for subsequent use;
[0035] (2) Send olmesartan medoxomil powder, silicified microcrystalline cellulose, pregelatinized starch, and croscarmellose sodium into the mixer for full mixing, and then send it into the mixer with magnesium stearate and titanium dioxide The total mixing is ...
Embodiment 2
[0040] A kind of olmesartan medoxomil amlodipine tablet, its prescription contains the following components by weight: 50 parts of olmesartan medoxomil, 12 parts of amlodipine besylate, 65 parts of silicified microcrystalline cellulose, 30 parts of pregelatinized starch 5 parts, 5 parts of croscarmellose sodium, 30 parts of calcium hydrogen phosphate, 5 parts of sodium carboxymethyl starch, 2 parts of magnesium stearate, 0.6 parts of titanium dioxide, 0.6 parts of talcum powder, 5 parts of coating premix .
[0041] The preparation method of described olmesartan medoxomil amlodipine sheet, comprises the following steps:
[0042] (1) Get olmesartan medoxomil and amlodipine besylate and pulverize through a 100-mesh sieve for subsequent use;
[0043] (2) Send olmesartan medoxomil powder, silicified microcrystalline cellulose, pregelatinized starch, and croscarmellose sodium into the mixer for full mixing, and then send it into the mixer with magnesium stearate and titanium dioxid...
Embodiment 3
[0048] Dissolution Determination
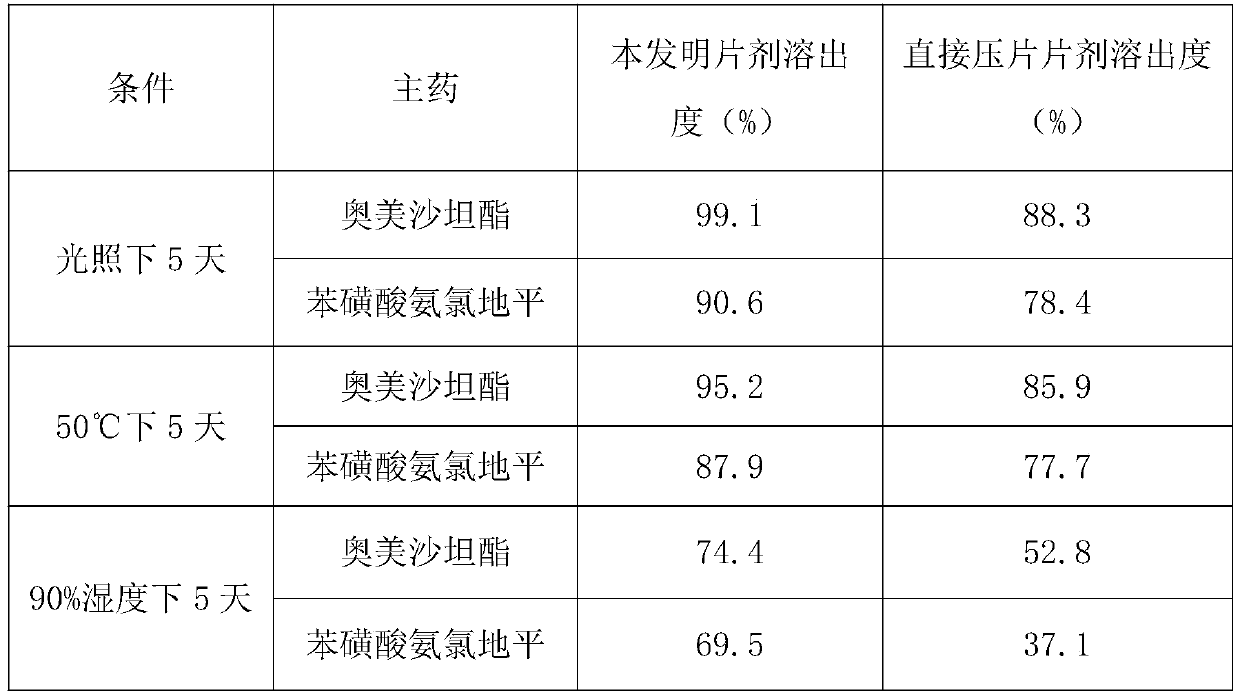
[0049] Investigate under the same time, the average dissolution rate of each 1000 pieces of olmesartan medoxomil and amlodipine tablets of the present invention and common direct compression tablet under light, high temperature and high humidity conditions, as shown in table 1 Shown:
[0050] Table 1 Dissolution Determination
[0051]
[0052]As can be seen from the above table, the olmesartan medoxomil amlodipine tablet of the present invention has better dissolution rate under different conditions than the olmesartan medoxomil amlodipine tablet of common direct compression, and it can be seen that the preparation process is more effective. Help to ensure the effectiveness of the tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com