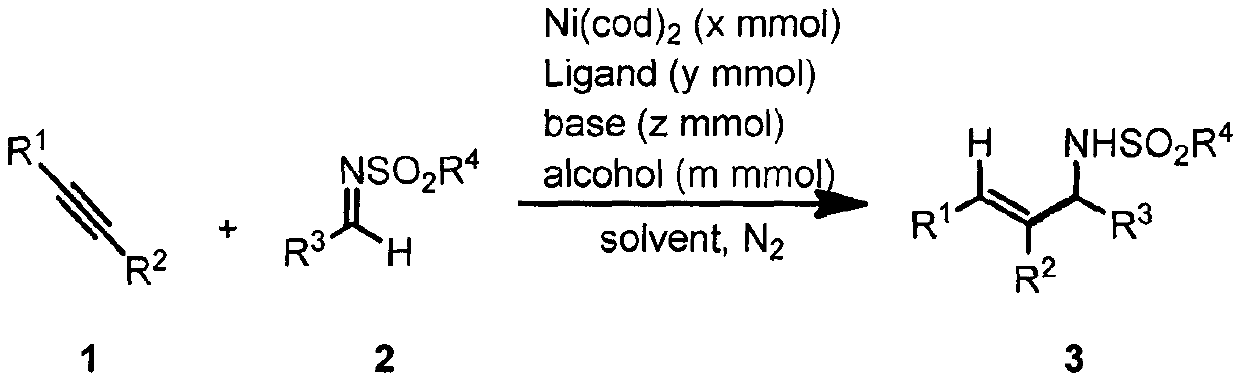Method for promoting reductive coupling reaction of imine and alkyne by means of alcohol to build allyl amine derivative
A technology for allylamine and derivatives, which is applied in the field of synthesis of allylamine derivatives, which can solve the problems of increased preparation costs, cumbersome steps, and strict operating requirements, and achieves convenient follow-up processing procedures, convenient operation and processing, and simple equipment requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of (E)-4-Methyl-N-(1,2,3-triphenylallyl)benzenesulfonamide
[0032]
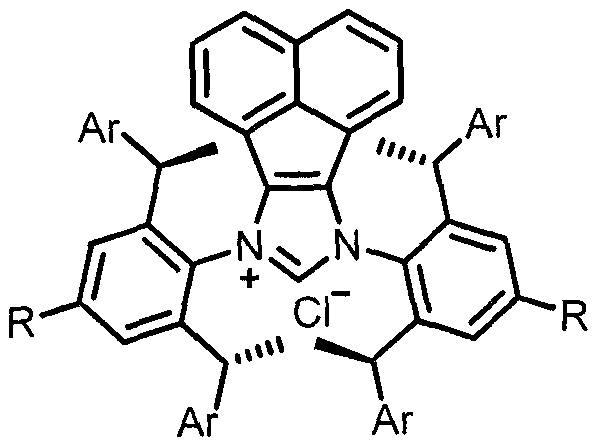
[0033] In a nitrogen atmosphere, the ligands AnIPr.HCl (5.5mg, 5mol%), t-BuOK (2.3mg, 10mol%), Ni(cod) were sequentially added to the reaction flask 2 (5.5mg, 10mol%), THF (1.0mL), i-PrOH (1.0mL), finally added raw materials 1a (35.6mg, 0.2mmol) and 2a (62mg, 0.24mmol), stirred at 100°C for 18 hours, cooled Return to room temperature, dilute with ethyl acetate, filter with celite, concentrate and separate by column chromatography to obtain the target product 3a as a white solid in a yield (81%). 1 H NMR (400MHz, CDCl 3 )δ7.69(d, J=8.4Hz, 2H), 7.29-7.24(m, 5H), 7.24-7.17(m, 3H), 7.13(t, J=7.6Hz, 2H), 7.09-6.99(m , 3H), 6.78-6.72(m, 2H), 6.8(d, J=6.8Hz, 2H), 6.48(s, 1H), 5.36(d, J=7.6Hz, 1H), 4.83(d, J= 7.6Hz, 1H), 2.36(s, 3H). 13 C NMR (100MHz, CDCl 3 ( C 28 h 29 N 2 o 2 S([M+NH 4 ] + ) 457.1944, Found 457.1942.
Embodiment 2
[0034]Example 2: Synthesis of (E)-2-Methyl-N-(1,2,3-triphenylallyl)propane-2-sulfonamide
[0035]
[0036] In a nitrogen atmosphere, the ligands AnIPr.HCl (5.5mg, 5mol%), t-BuOK (2.3mg, 10mol%), Ni(cod) were sequentially added to the reaction flask 2 (5.5mg, 10mol%), THF (1.0mL), i-PrOH (1.0mL), finally added raw materials 1a (35.6mg, 0.2mmol) and 2b (54mg, 0.24mmol), stirred at 100°C for 18 hours, cooled Return to room temperature, dilute with ethyl acetate, filter with diatomaceous earth, concentrate and separate by column chromatography to obtain the target product 3b as a white solid, yield (65%). 1 H NMR (400MHz, CDCl 3 ( d, J=6.8Hz, 2H), 6.78(s, 1H), 5.52(d, J=10.0Hz, 1H), 4.29(d, J=9.6Hz, 1H), 1.37(s, 9H). 13 C NMR (100MHz, CDCl 3 )δ141.7, 140.2, 137.4, 136.1, 129.7, 129.6, 129.4, 128.9, 128.8, 128.1, 128.0, 127.8, 127.3, 127.1, 65.5, 60.3, 24.3. HRMS (ESI) calcd.for C 25 h 31 N 2 o 2 S ([M+NH 4 ] + ) 423.2101, Found 423.2101.
Embodiment 3
[0037] Example 3: Synthesis of (E)-N-(2,3-diphenyl-1-(p-tolyl)allyl)-4-methylbenzenesulfonamide
[0038]
[0039] In a nitrogen atmosphere, the ligands AnIPr.HCl (5.5mg, 5mol%), t-BuOK (2.3mg, 10mol%), Ni(cod) were sequentially added to the reaction flask 2 (5.5mg, 10mol%), THF (1.0mL), i-PrOH (1.0mL), finally added raw materials 1a (35.6mg, 0.2mmol) and 2c (65.5mg, 0.24mmol), stirred at 100°C for 18 hours, Cool to room temperature, dilute with ethyl acetate, filter with celite, concentrate and separate by column chromatography to obtain the target product 3c as a white solid in a yield (79%). 1 HNMR (400MHz, CDCl 3 )δ7.69 (d, J=8.4Hz, 2H), 7.24-7.17 (m, 3H), 7.17-7.10 (m, 4H), 7.10-7.01 (m, 5H), 6.79-6.72 (m, 2H) , 6.69(d, J=6.8Hz, 2H), 6.48(s, 1H), 5.31(d, J=8.0Hz, 1H), 4.77(d, J=8.0 Hz, 1H), 2.36(s, 3H) , 2.32(s, 3H). 13 C NMR (100MHz, CDCl 3 )δ143.4, 139.9, 137.7, 137.6, 137.5, 136.2, 136.1, 129.8, 129.6, 129.4, 129.4, 129.3, 128.8, 127.9, 127.8, 127.5, 127.3, 127....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com