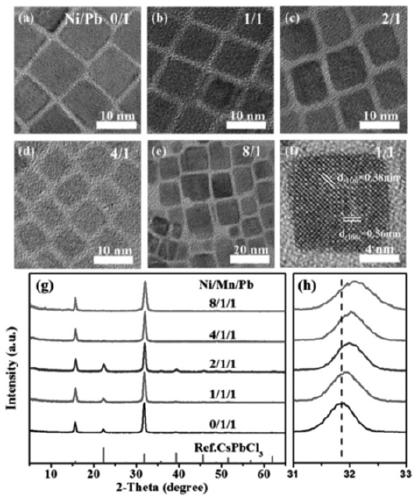
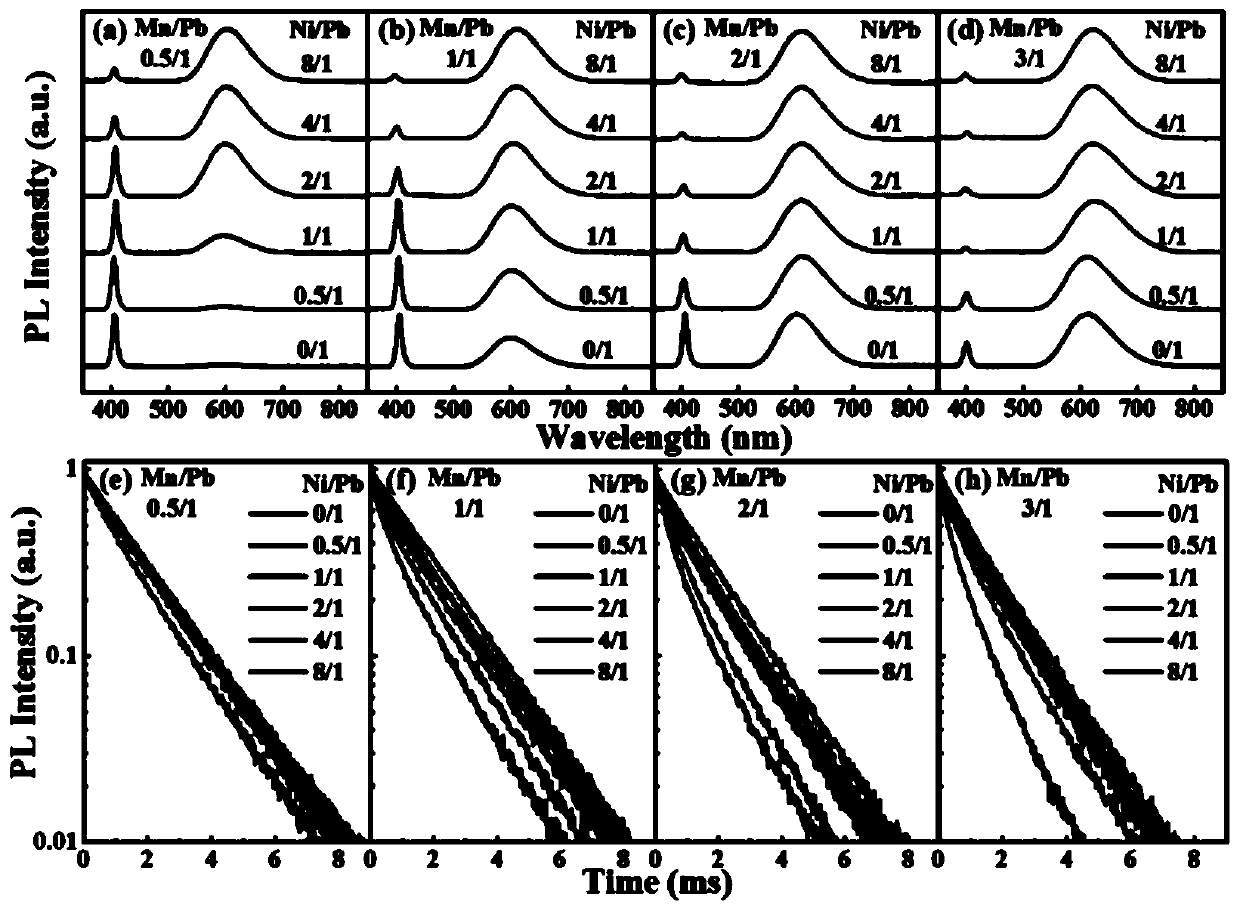
Method for increasing manganese doping concentration and light emission efficiency of manganese-doped CsPbCl3 nanocrystal
A technology of luminous efficiency and manganese doping, applied in nano optics, nanotechnology, luminescent materials, etc., to achieve the effect of improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The present invention provides a kind of by introducing NiCl 2 Improve Mn-doped CsPbCl 3 A preparation method for doping and luminous efficiency of nanocrystals, the method comprising:
[0029] Step 1: Mix cesium carbonate, oleic acid and an oil phase solvent, and under the protection of an inert gas, stir and heat until the solution is clarified to obtain a cesium precursor solution, which is cooled to 100°C for use. The molar mass of the cesium carbonate is 0.68mmol, and the oil phase solvent is preferably one or more of octadecylamine, octadecene, oleylamine, more preferably octadecene; oleic acid and oil phase solvent The volume ratio is preferably 1:(6~10), more preferably 1:8; the inert gas is preferably nitrogen, helium, neon or argon, more preferably argon; the heating temperature is 130 ~170°C, more preferably 150°C for 5 minutes.
[0030] Step 2: Mix octadecene, oleic acid, oleylamine, trioctylphosphine, lead chloride, manganese chloride and nickel chloride...
Embodiment 1
[0035] Step 1: join 0.68mmol cesium carbonate, 1.25mL oleic acid, 10mL octadecene in the three-necked bottle of 50mL at room temperature, vacuumize, feed inert gas, to remove gas, under the protection of argon, will Stir and heat the mixed solution to 150°C, and keep this temperature until the solution is clear and transparent to obtain a cesium precursor solution, and cool it to 100°C for use; the number of vacuum pumping can be multiple times, specifically 2, 3, or 4 times Wait;
[0036] Step 2: Add 5mL octadecene, 1.5mL oleic acid, 1.5mL oleylamine, 1mL trioctylphosphine, 0.2mmol lead chloride, 0.2mmol manganese chloride and 0.2mmol nickel chloride into a 50mL three-necked bottle, Vacuumize and introduce inert gas to remove gas. Under the protection of argon, stir and heat the mixed solution to 190°C, and keep this temperature for 10min to fully dissolve nickel chloride; the number of times of vacuuming can be multiple, Specifically, it can be 2 times, 3 times, 4 times, et...
Embodiment 2
[0040] Step one: at room temperature, 0.68mmol cesium carbonate, 1.25mL oleic acid, 8mL octadecylamine are added in the three-necked bottle of 50mL, vacuumize, feed inert gas, to remove gas, under the protection of nitrogen, will The mixed solution is stirred and heated to 130°C, and kept at this temperature until the solution is clear and transparent to obtain a cesium precursor solution, which is cooled to 100°C for use; the number of vacuum pumping can be multiple times, specifically 2, 3, or 4 times Wait;
[0041] Step 2: Add 5mL octadecene, 1.5mL oleic acid, 1.5mL oleylamine, 1mL trioctylphosphine, 0.2mmol lead chloride, 0.2mmol manganese chloride and 0.2mmol nickel chloride into a 50mL three-necked bottle, Vacuumize and introduce inert gas to remove gas. Under the protection of nitrogen, stir and heat the mixed solution to 190°C, and keep this temperature for 10min to fully dissolve nickel chloride; the number of times of vacuuming can be multiple, the specific It can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com