Application of sequence-optimized secreted form FAPalpha-based tumor DNA vaccine
A DNA vaccine and vaccine technology, applied in the field of tumor DNA vaccine, to achieve the effect of good protein expression level, high safety and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
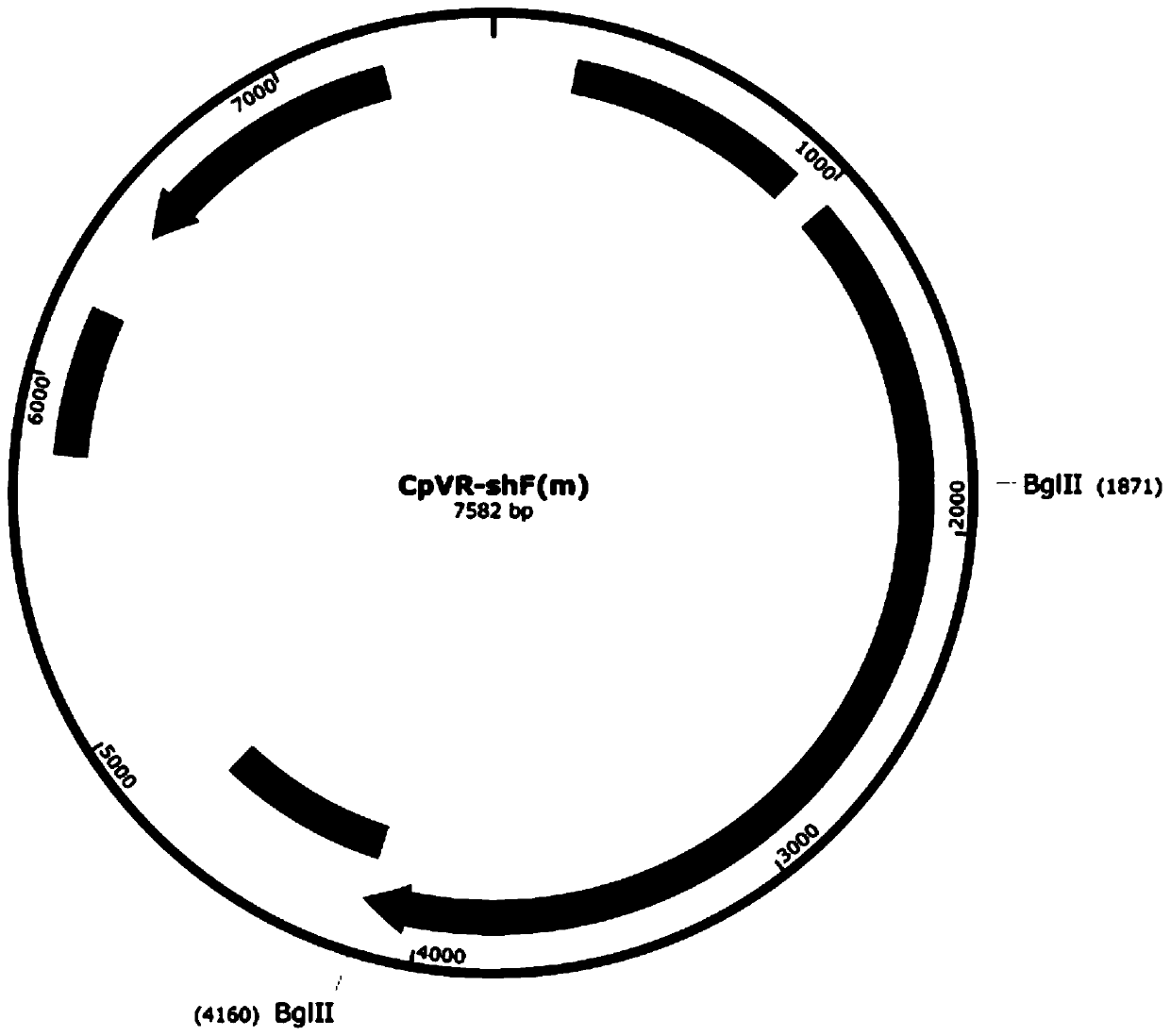
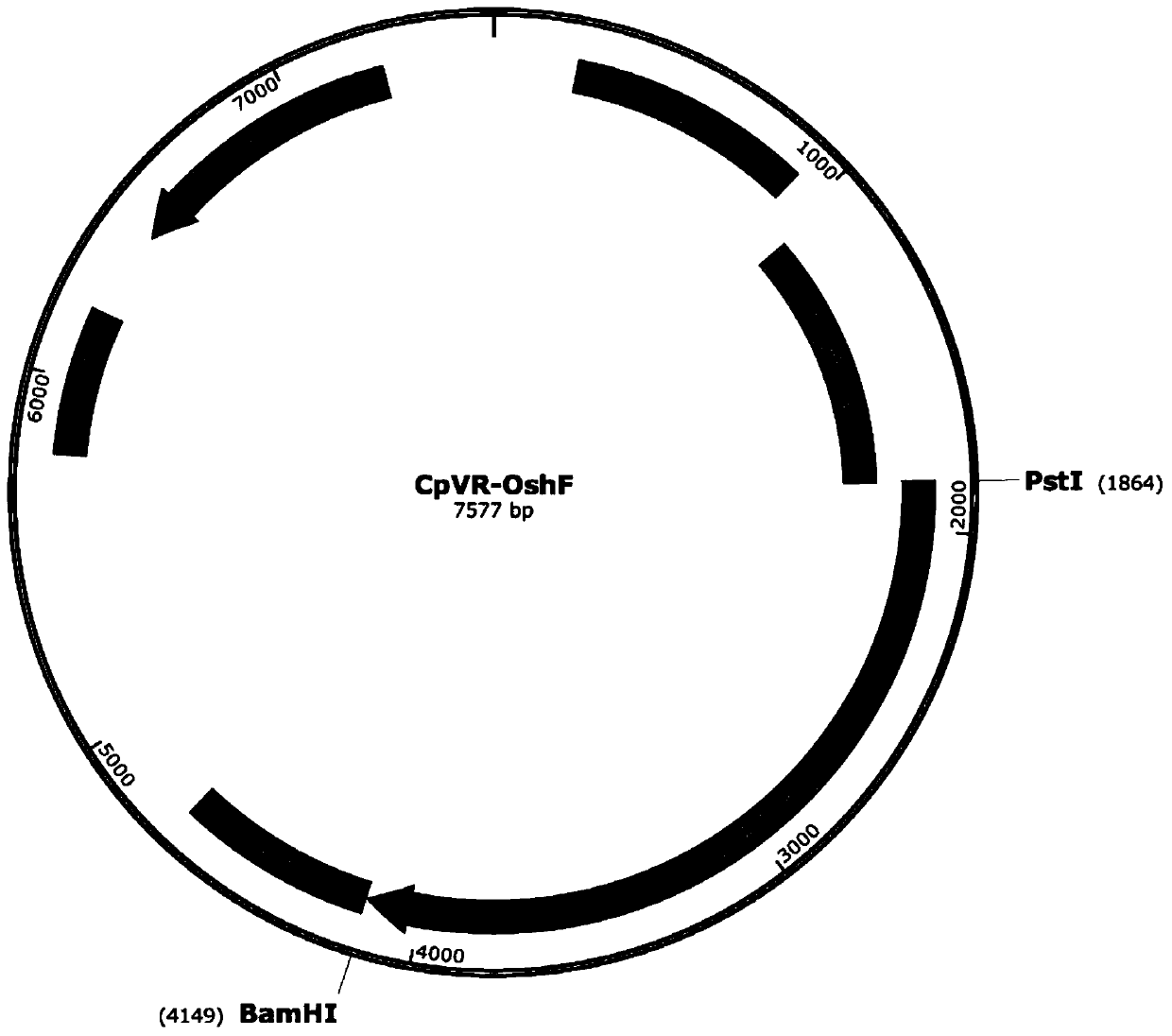
[0033] 1. Construction and identification of CpVR-shF(m) and CpVR-OshF(m) DNA vaccines
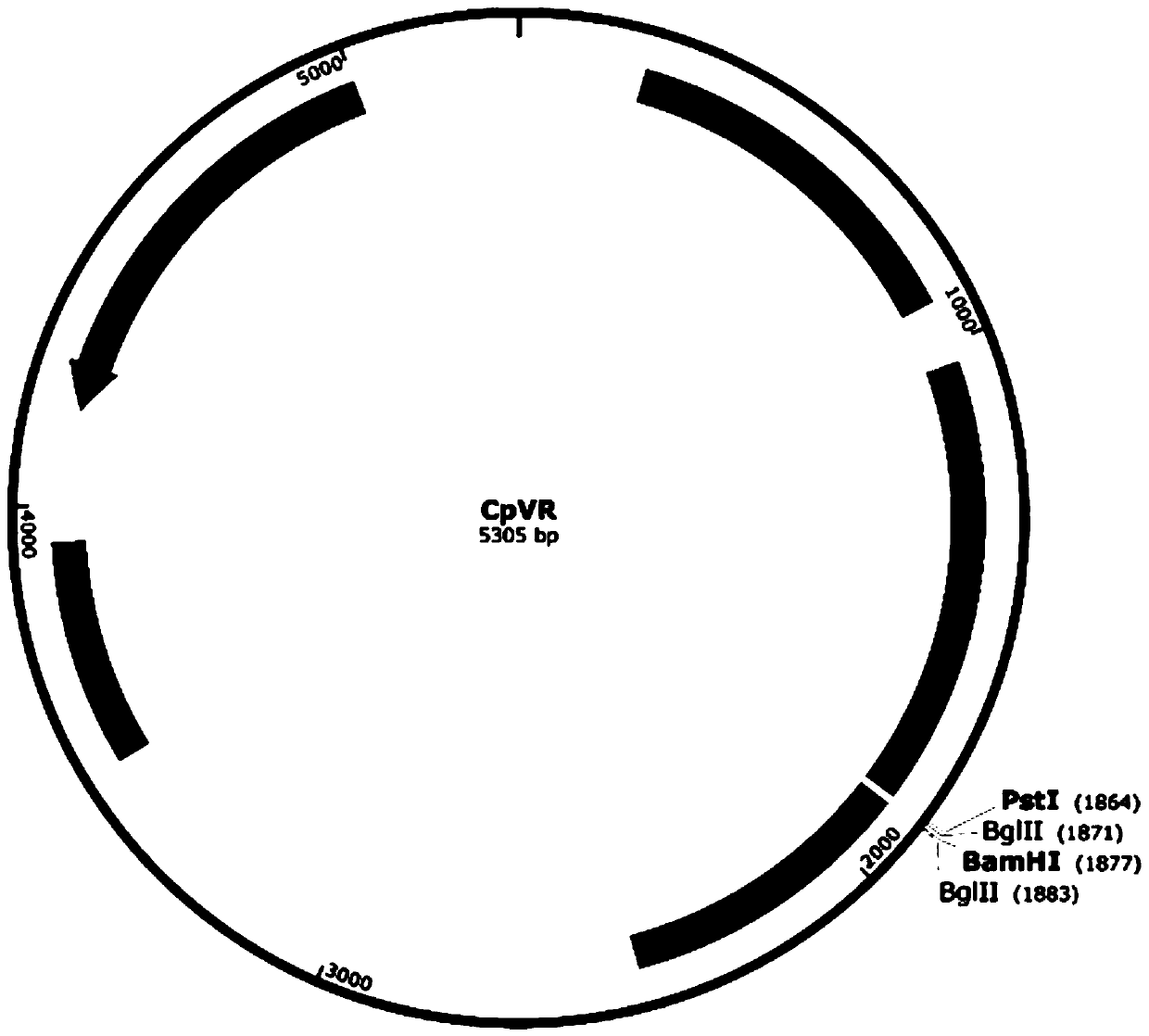
[0034] The vector used is the eukaryotic expression plasmid VR1012 with the introduction of CpG motif, referred to as CpVR, which contains promoter CMV, intron A, BGH sequence, CpG sequence, kanamycin resistance sequence, a total of 5305bp, see the map Figure 1A .
[0035] Using CpVR-hF(m) (and CpVR-FAP) as a template, the nucleotide sequence SEQ ID NO: 1 was obtained by multi-step PCR, and BglII restriction sites and homologs identical to the sequences at both ends of the vector were introduced at both ends. For the source arm sequence, perform DNA electrophoresis and gel recovery on the target DNA fragment and the vector fragment obtained by digestion with BglII to obtain the target fragment and vector, use a homologous recombination kit, incubate at 50°C for 15 minutes, transform Escherichia coli, and coat the card Namycin-resistant LB plates were incubated overnight at 37°C. Select p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com