Composite chlorine dioxide preparation method
A chlorine dioxide and chlorine source technology, applied in botany equipment and methods, chemicals for biological control, animal repellants, etc., can solve problems such as prone to explosion, high chlorine content, and excessive chlorate , to reduce the risk of decomposition and explosion, reduce the reaction temperature and improve the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The second aspect of the present invention provides a kind of preparation method of composite chlorine dioxide, comprises the following steps:
[0044] A. take by weighing the preparation raw material of above-mentioned composite chlorine dioxide;
[0045] b. Dissolve chlorate and chlorine source completely in water, make solution A;
[0046] c. Mix reducing agent and acidity control agent, be made into solution B;
[0047] d. Under the condition of negative pressure, mix solution A and solution B evenly in the reaction kettle, and the reaction temperature is 50-80°C.
[0048] In some preferred embodiments, the preparation method of said composite chlorine dioxide comprises the following steps:
[0049] A. take by weighing the preparation raw material of above-mentioned composite chlorine dioxide;
[0050] b. Dissolve chlorate and chlorine source completely in water, make solution A;
[0051] c. Mix reducing agent and acidity control agent, be made into solution B; ...
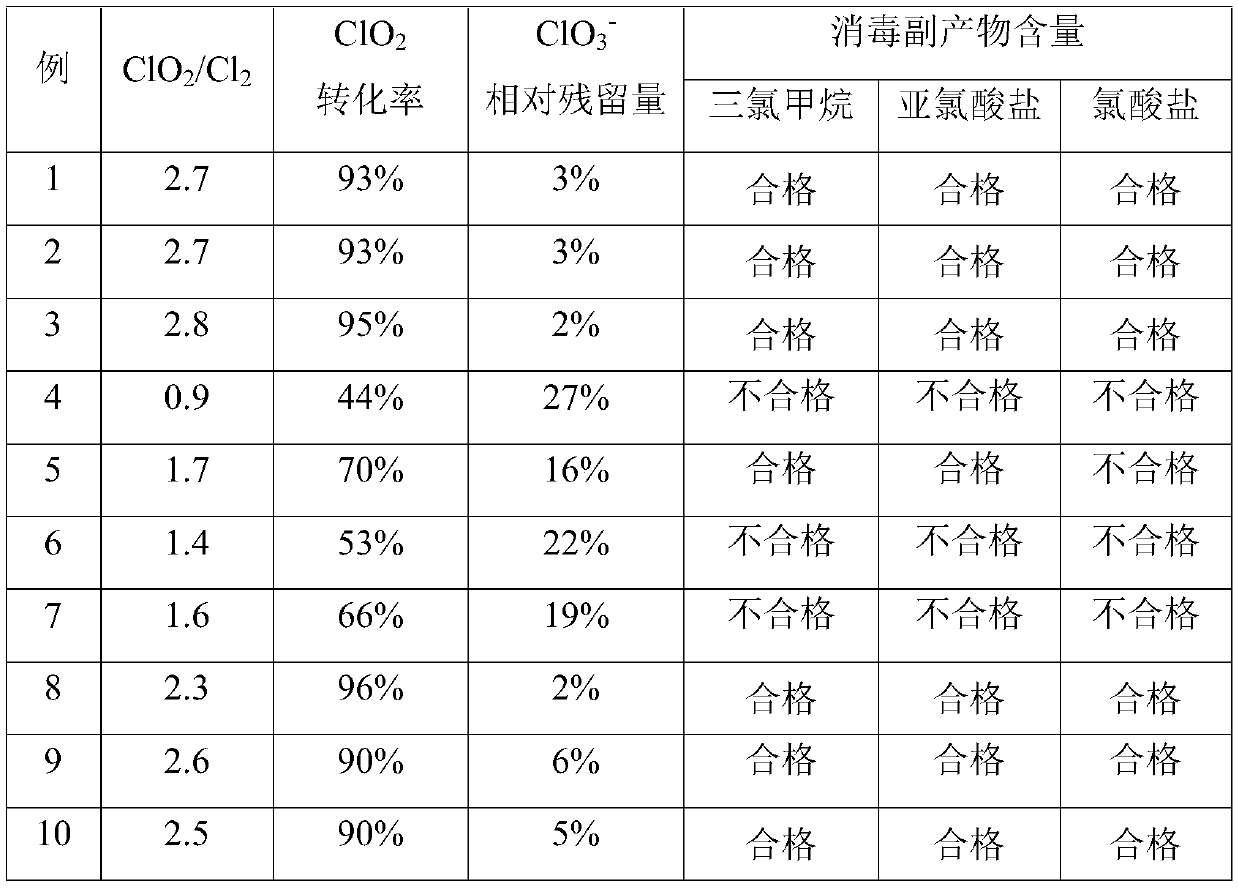
Embodiment 1
[0062] Example 1 provides a raw material for the preparation of composite chlorine dioxide, which comprises 1 part of chlorate, 0.5 part of chlorine source, 0.2 part of reducing agent, 2.5 parts of acidity control agent and 3 parts of water in parts by weight.
[0063] The chlorate is sodium chlorate (CAS number: 7775-09-9).
[0064] The chlorine source is inorganic chloride; the inorganic chloride is sodium chloride (CAS number: 7647-14-5).
[0065] The reducing agent is a reducing organic acid; the reducing organic acid is oxalic acid (CAS number: 144-62-7).
[0066] The acidity control agent is a strong inorganic acid; the strong inorganic acid is sulfuric acid (CAS number: 7664-93-9); the concentration of the sulfuric acid is 65 wt%.
[0067] This example also provides a kind of preparation method of composite chlorine dioxide, comprises the following steps:
[0068] A. take by weighing the preparation raw material of above-mentioned composite chlorine dioxide;
[0069]...
Embodiment 2
[0073] Embodiment 2 provides a preparation raw material for composite chlorine dioxide, which comprises 1 part of chlorate, 0.5 part of chlorine source, 0.2 part of reducing agent, 2.5 parts of acidity control agent and 3 parts of water in parts by weight.
[0074] The chlorate is potassium chlorate (CAS number: 3811-04-9).
[0075] The chlorine source is inorganic chloride; the inorganic chloride is potassium chloride (CAS number: 7447-40-7).
[0076] The reducing agent is a reducing organic acid; the reducing organic acid is oxalic acid.
[0077] The acidity control agent is a strong inorganic acid; the strong inorganic acid is sulfuric acid; the concentration of the sulfuric acid is 65wt%.
[0078] This example also provides a preparation method of composite chlorine dioxide, which is similar to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com