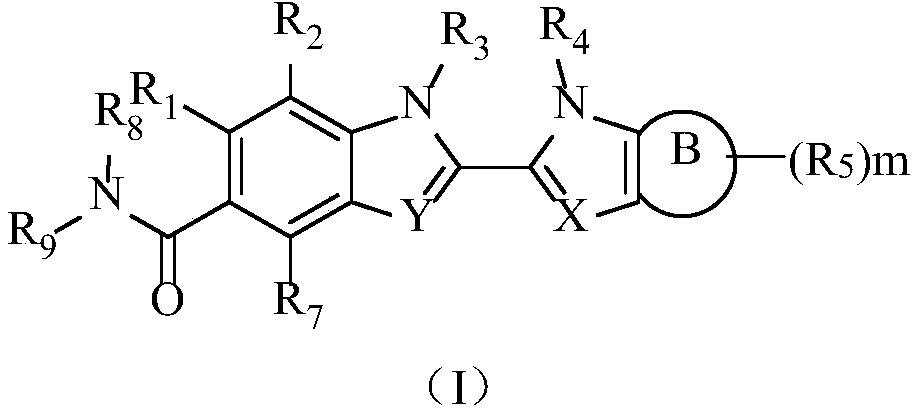
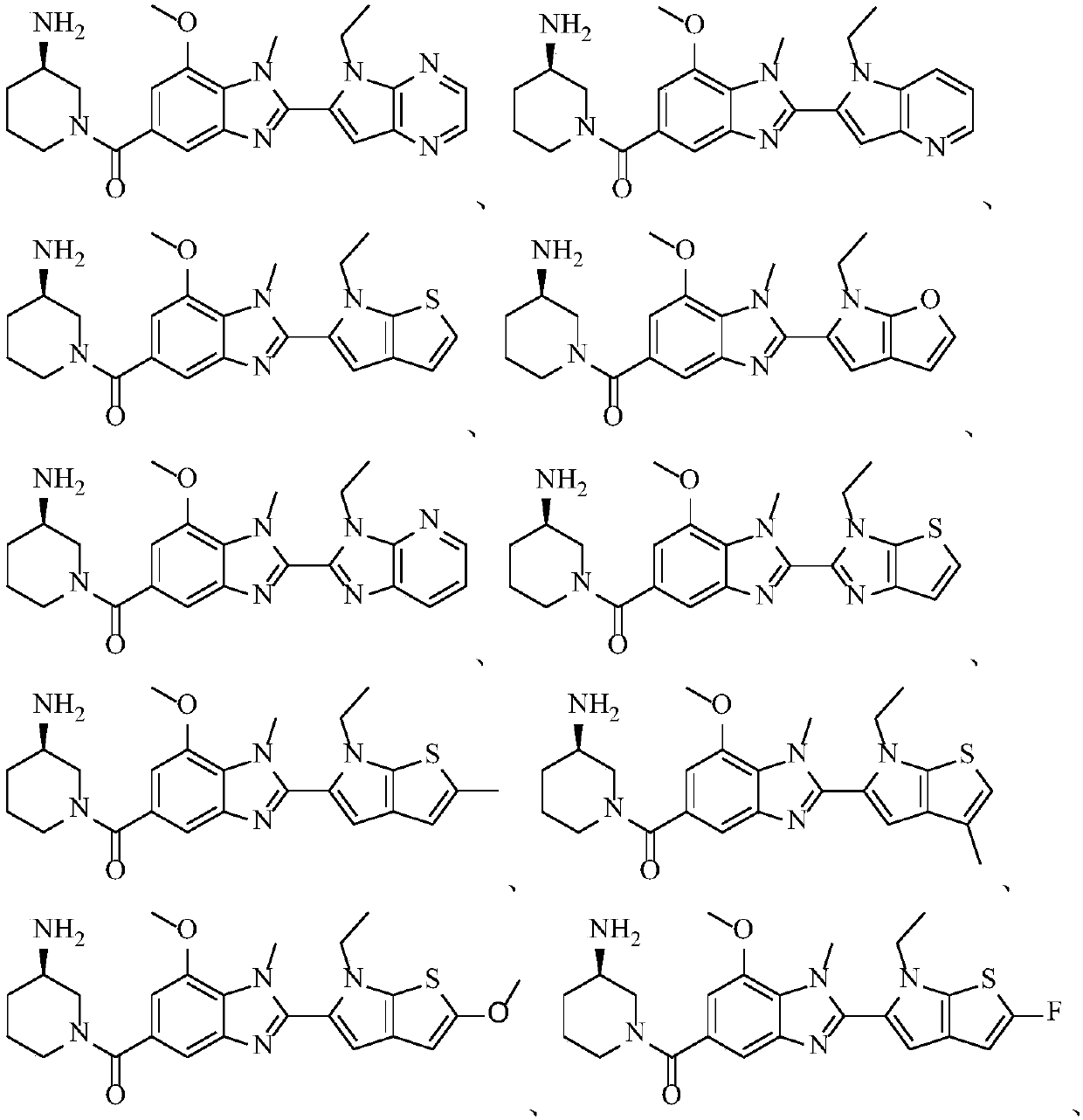
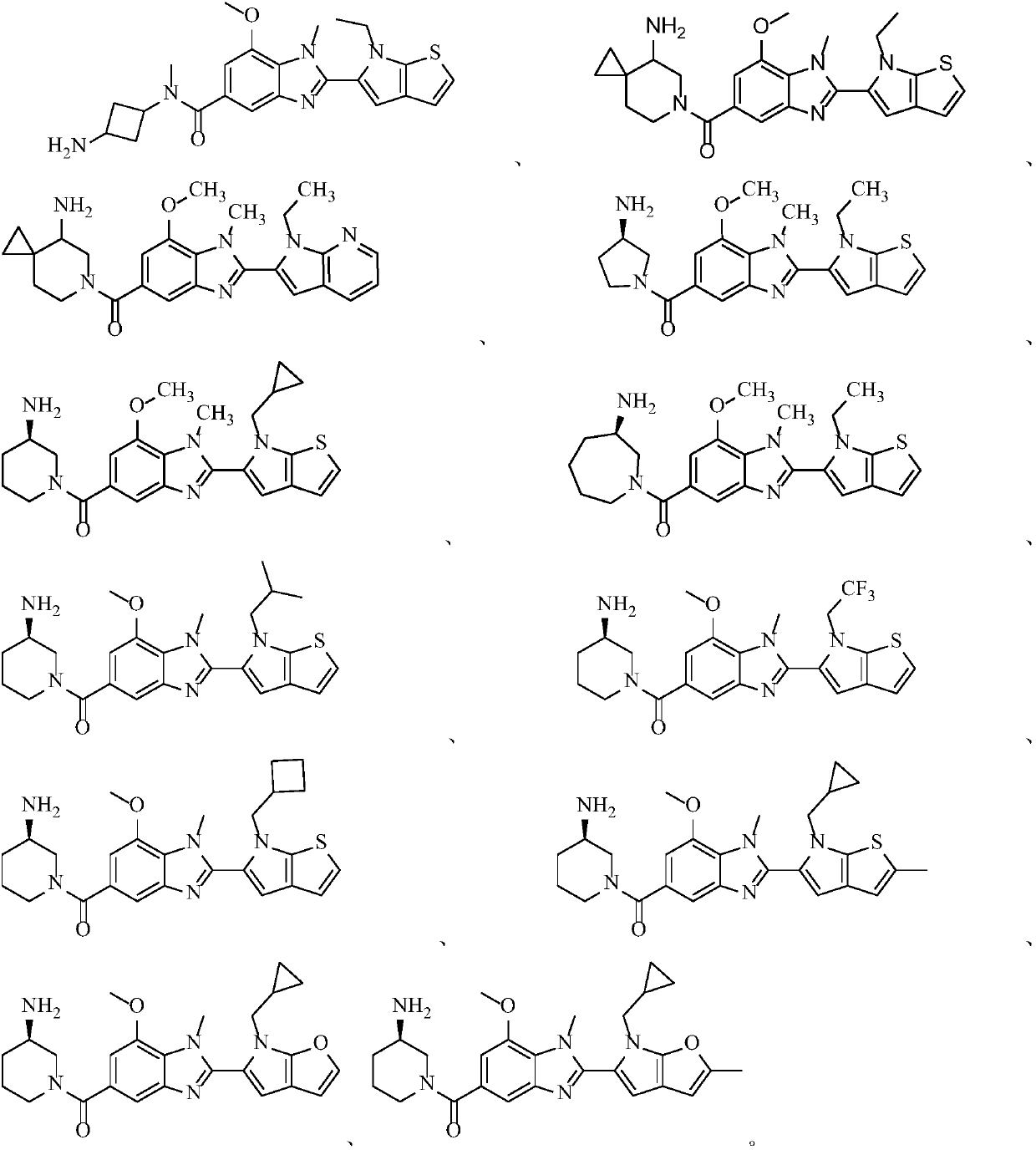
Peptidylarginine deiminase inhibitor and application thereof
An amino and alkyl technology, applied in the field of medicine, can solve the problem of the lack of varieties of PAD4 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0398] Embodiment 1: Synthesis of intermediate 3-methoxy-4-(methylamino)-5-nitrobenzoic acid methyl ester
[0399]
[0400] Step 1: Synthesis of methyl 4-hydroxy-3-methoxy-5-nitrobenzoate
[0401]
[0402] Methyl 4-hydroxy-3-methoxybenzoate (25.4g, 0.139mol) was dissolved in glacial acetic acid (100mL), and a mixed solution of nitric acid (10.2mL) and acetic acid (52mL) was slowly added dropwise under ice-cooling, about 1 The hour dropwise addition was completed. The reaction solution was slowly raised to room temperature and stirred for 4 hours. After filtering, the filter cake was washed successively with water, n-hexane and diethyl ether, and dried to obtain the product as a yellow solid (21.0 g, yield: 66%).
[0403] Step 2: Synthesis of methyl 4-chloro-3-methoxy-5-nitrobenzoate
[0404]
[0405] Dissolve methyl 4-hydroxy-3-methoxy-5-nitrobenzoate (21g, 0.095mol) in DMF (200mL), cool to -20°C, slowly add oxalyl chloride (23.7mL) dropwise, dropwise The temperatur...
Embodiment 2
[0410] Example 2: Synthesis of intermediate 1-ethyl-1H-pyrrolo[3,2-b]pyridine-2-carbaldehyde
[0411]
[0412] Step 1: Synthesis of 1-(phenylsulfonyl)-1H-pyrrolo[3,2-b]pyridine
[0413]
[0414] Under nitrogen protection, sodium hydride (mass fraction 60%, 0.8g, 20mmol, 2.0eq) was added in batches to anhydrous THF (30mL) at room temperature, and 1H-pyrrolo[3,2-b]pyridine (1.19 g, 10mmol, 1.0eq) was dissolved in anhydrous THF (10mL), slowly added dropwise to the system, after the drop was completed, the reaction was incubated for 30 minutes. Benzenesulfonyl chloride (3.5g, 20mmol, 2.0eq) was dissolved in THF (10mL), slowly added dropwise to the system, and the dropwise addition was completed in 15 minutes, and the reaction was carried out at room temperature for 4 hours. LC-MS detected that the reaction was complete, slowly added 0.1mol / L HCl aqueous solution (20mL) dropwise to quench the reaction, added water (30mL), extracted with EA (50mL×2), combined the organic phas...
Embodiment 3
[0426] Example 3: Synthesis of intermediate 5-ethyl-5H-pyrrolo[2,3-b]pyrazine-6-carbaldehyde
[0427]
[0428] Step 1: Synthesis of 3-((trimethylsilyl)ethynyl)pyrazin-2-amine
[0429]
[0430] Under nitrogen protection, 3-chloropyrazin-2-amine (12.9g, 0.1mol, 1.0eq) was dissolved in DMF (150mL), CuI (9.5g, 0.05mol, 0.5eq), tetrakis(triphenyl Phosphine) palladium (11.55g, 0.01mol, 0.1eq) and triethylamine (30.3g, 0.3mol, 3.0eq), cool down to 0°C, slowly add trimethylsilylacetylene (14.7g, 0.15mol, 1.5 eq), after the dropwise addition was completed, it was heated to 70° C. and stirred for 12 hours. TLC and LC-MS detected that the reaction was complete. Pour the reaction solution into water (300mL), add EA (500mL), filter with suction, wash the filter cake with EA (50mL×2), separate the filtrate, and wash the organic phase with saturated brine , dried, filtered with suction, the filtrate was concentrated, and the crude product was purified by silica gel column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com