Umbilical cord mesenchymal stem cells (MSCs) and culture method and application thereof
A kind of stem cell and culture method technology, applied in the field of umbilical cord mesenchymal stem cell MSCs and its culture, can solve the problems of hindering the clinical treatment of stem cells, cell mass embolism, stem cell adhesion, etc., to improve the ability of cell proliferation and clone formation, Enhances adaptability and reduces the effect of clumping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A kind of MSC stem cell obtained by cultivating by the following method:
[0053] 1. Donor Screening
[0054] 1. Donors should draw blood within one month before sample collection for detection of severe infectious diseases, including but not limited to: HIV (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum (TP), giant cells Virus (CMV), Epstein-Barr virus, phagocytic T-cell virus (HLTV), any one of the seven infectious disease tests showing that the donors carry pathogens (including Treponema pallidum previous infection) are excluded;
[0055] 2. The donor draws blood within one month before sample collection for DNA testing of serious genetic diseases, including but not limited to: achondroplasia, congenital deafness, vitamin D-resistant rickets, phenylketonuria, hemophilia, progressive Muscular dystrophy, glucose 6-phosphate dehydrogenase (G-6-PD) deficiency (faba bean disease), etc.; tested positive, are excluded);
[0056] 3. The donor ...
Embodiment 2
[0096] A kind of MSCs stem cell injection, obtained by the following method:
[0097] 1. Preparation:
[0098] 1.1 Turn on the water bath for rewarming to stabilize the water temperature at 37°C;
[0099] 1.2 The biosafety cabinet is sterilized and ventilated by ultraviolet light 30 minutes in advance;
[0100] 1.3 Prepare normal saline according to the number of recovered cells and add it to the centrifuge tube. The volume of normal saline should be at least 10 times the volume of the whole cryopreservation solution;
[0101] 2. Information check:
[0102] Take out the frozen cells from the liquid nitrogen tank according to the predetermined requirements, and carefully check key information such as cell type, cell code, cell number, and cell generation to prevent errors.
[0103] 3. Immediately put the frozen cells taken out of the liquid nitrogen tank into the rewarmed water bath to resuscitate for 2 minutes until the frozen storage solution is completely melted, keep sha...
Embodiment 3
[0118] An application of MSCs stem cell injection in the treatment of myocardial infarction.
[0119] The umbilical cord mesenchymal stem cell MSCs preparation of the present invention has a wide range of applications. Currently, we have formulated the MSCs of Example 1 into a composite preparation as shown in Example 2 and applied it to the treatment of patients with myocardial infarction to observe the curative effect.
[0120] 1. Observe the morphology of peripheral blood cells under a microscope.
[0121] 1. Method
[0122] The peripheral blood of subjects in the control group (the MSC stem cell culture was not added Ligustrazine hydrochloride and Shenmai injection) and the administration group (composite preparation of Example 2) was observed under a microscope.
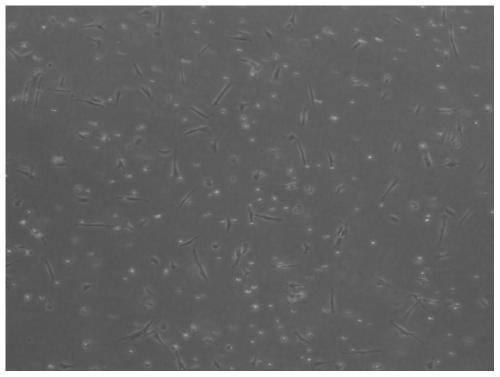
[0123] 2. Results
[0124] The result is as Figure 10-11 as shown, Figure 10 Peripheral blood condition of the subjects in the control group, Figure 11 Peripheral blood condition of subjects in the admin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com