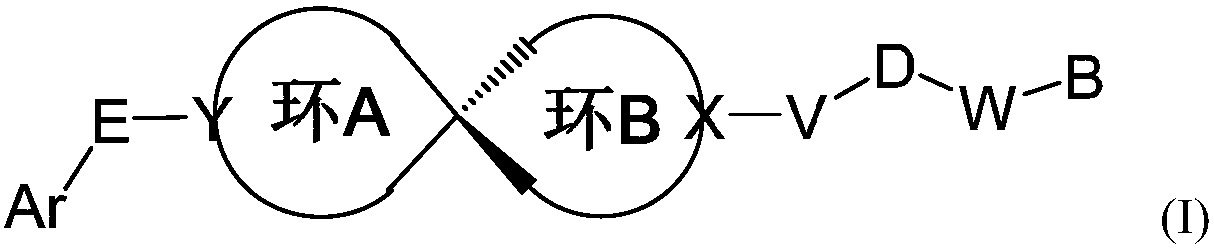
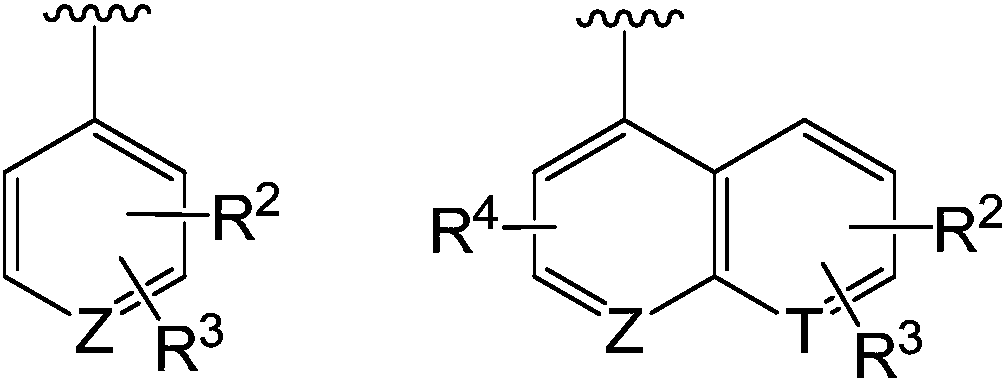
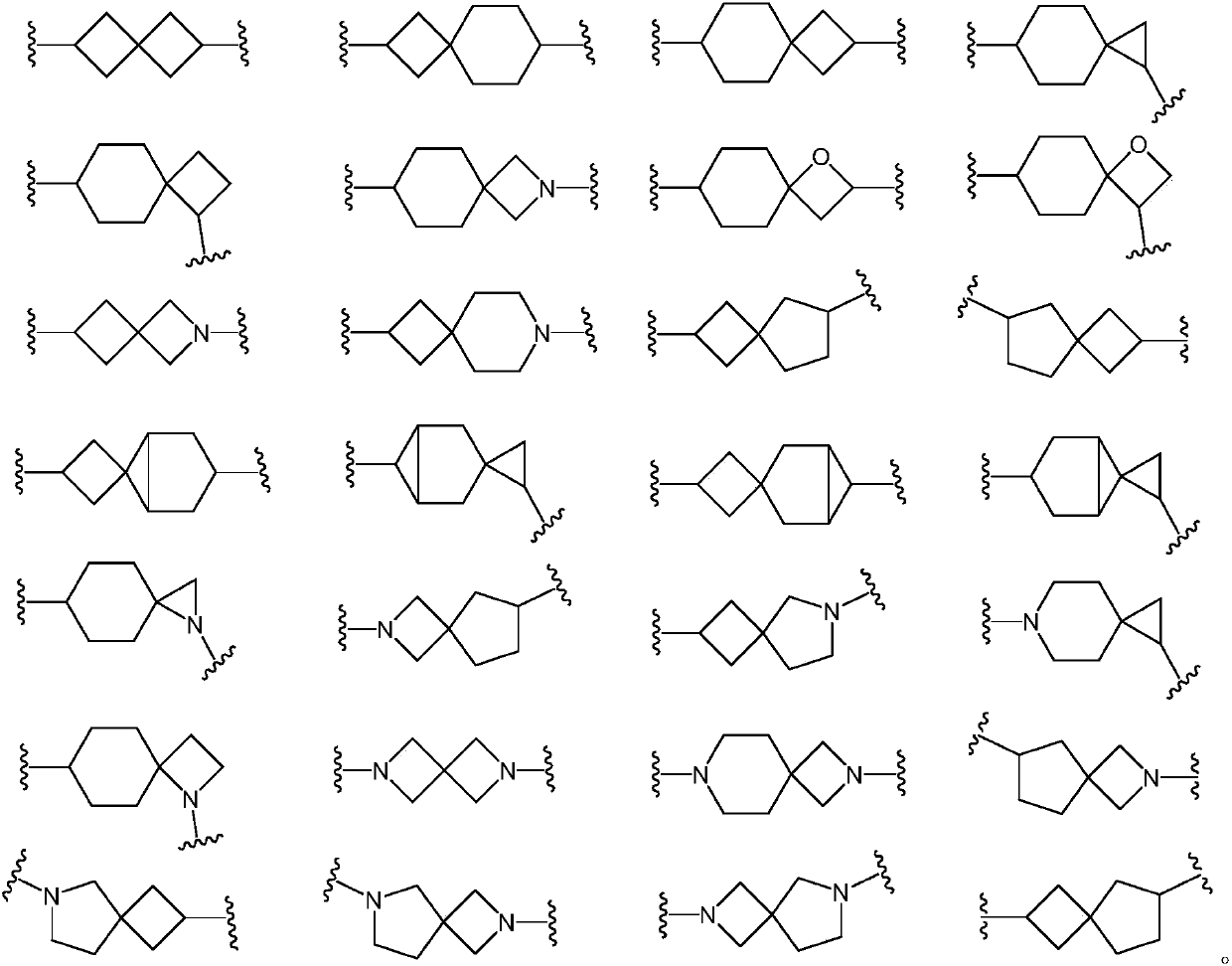
Spiro compound taking as indoleamine-2, 3-dioxygenase inhibitor
A compound, C3-C6 technology, applied to the active ingredients of heterocyclic compounds, drug combination, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: N-(4-chlorophenyl)-6-(quinolin-4-yl)spiro[3.3]heptane-2-carboxamide (compound 1)
[0148]
[0149] The first step: (1-hydroxymethyl-3,3-dimethoxycyclobutyl)methanol
[0150]
[0151] Lithium aluminum hydride (28.81g, 759.7mmol) was added in batches to a three-necked flask containing tetrahydrofuran (1500mL), and then slowly added dropwise with isopropyl 3,3-dimethoxycyclobutane-1,1-dicarboxylate (99.57g, 345.3mmol) in tetrahydrofuran (500mL). Stirring at room temperature for 12h, TLC showed that the reaction was complete. Add saturated sodium potassium tartrate solution (27 mL) dropwise under ice-cooling to quench, and stir at room temperature for 1 h. After suction filtration, the filter cake was washed with dichloromethane / methanol (10:1, 200 mL×5), the organic phase filtrates were combined, and spin-dried to obtain a colorless and transparent oily product (59.8 g, yield 95%).
[0152] 1 H NMR (400MHz, CDCl 3 ): δ1.94(s,4H),3.12(s,6H),3.70(s,4H,)...
Embodiment 2
[0191] The preparation of embodiment 2 (±) (cis / trans) N-(4-chlorophenyl)-6-(quinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0192]
[0193] The first step: ethyl 2-(1,4-dioxaspiro[4.5]dec-8-ylidene) ethyl acetate
[0194]
[0195] Under ice-cooling, dissolve sodium hydrogen (52mg, 0.88mmol) and triethyl phosphonoacetate (52mg, 0.83mmol) in 5mL of N,N-dimethylformamide, stir for half an hour, then add compound 1,4 -Dioxaspiro[4.5]dec-8-one (100 mg, 0.65 mmol) was dissolved in 2 mL of N,N-dimethylformamide and added dropwise to the reaction solution, and stirring was continued at room temperature for 1 hour. Quenched by adding 50mL, extracted with ethyl acetate (20mL x 3), combined organic layers, washed with brine, dried over anhydrous sodium sulfate, filtered, spin-dried, and passed through a column (PE:EA=3:1) to obtain a white oil (128mg ,88%). 1 H NMR (400MHz, CDCl3): δ5.60(s,1H),4.05-4.11(m,2H),3.91-3.92(d,4H),2.95(t,3H),2.31(t,3H),1.67 -1.73(m,4H),1.19-1.23(m,3H)...
Embodiment 3
[0225] activity test
[0226] (1) Induced expression and purification method of IDO protein
[0227] First, the IDO gene was amplified by PCR, and the amplified PCR product was recovered, and then the pET28a plasmid (purchased from Shanghai Baoman Biotechnology Co., Ltd.) and the product recovered from IDO gel were digested with two restriction enzymes, EcoR I and Xho I. (37°C, enzyme digestion for 2h), gel run, recovery, T4 ligase linked overnight ligation product was added to DH5α competent, placed on ice for 30min, heat shock at 42°C for 90s, shake the plate, pick a single clone, PCR identification , Sequencing identification, all correct, that is, the pET28a-IDO plasmid was successfully constructed.
[0228] Shake the constructed BL21 containing the pET28a-IDO plasmid at 37°C to OD 600 0.6-0.8, add hemin (hemin) and 0.5mM IPTG (isopropyl-β-D-thiogalactoside) to a final concentration of 40 μM, induce at 16°C for 20h; after induction, at 4°C , 6000rpm centrifugation to coll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com