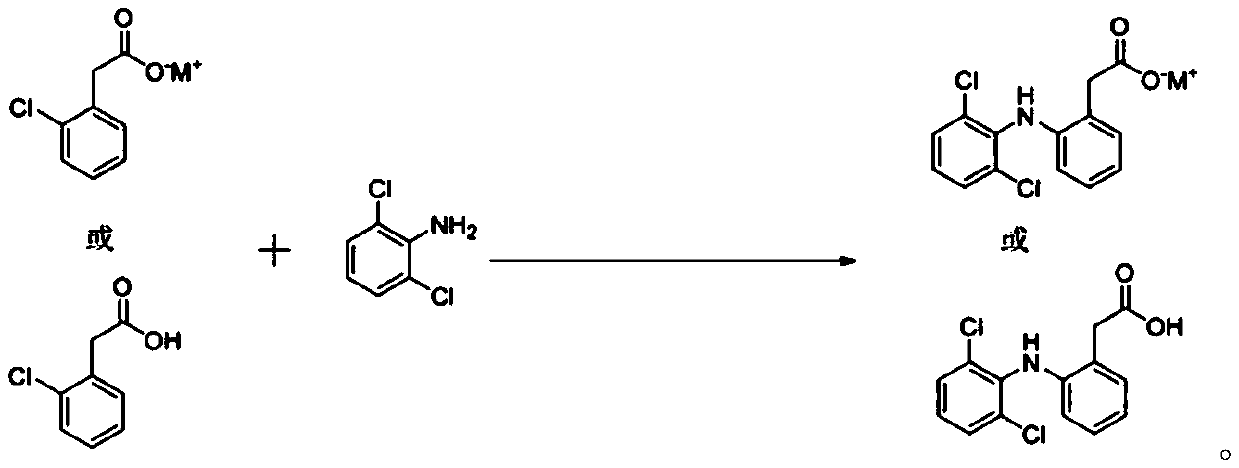
Preparation method of diclofenac
A technology of diclofenac and chlorophenylacetic acid is applied in the preparation of organic compounds, chemical instruments and methods, preparation of cyanide reaction, etc., can solve the problems of large amount of catalyst, large environmental pollution, many industrial three wastes, etc., and achieves high catalytic efficiency, High catalytic activity and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
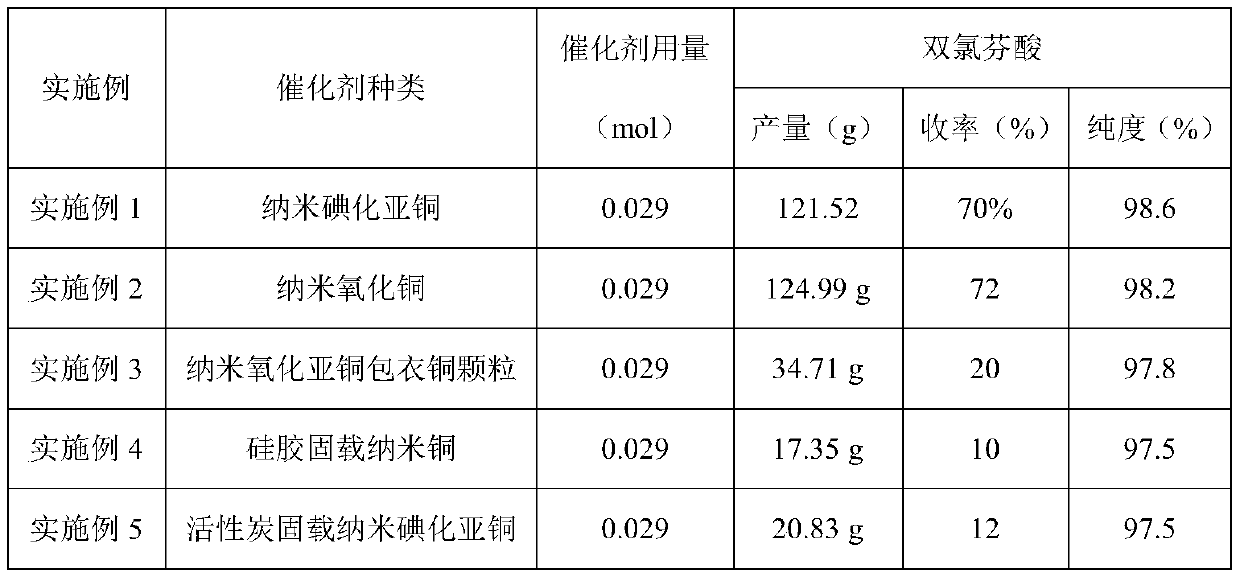
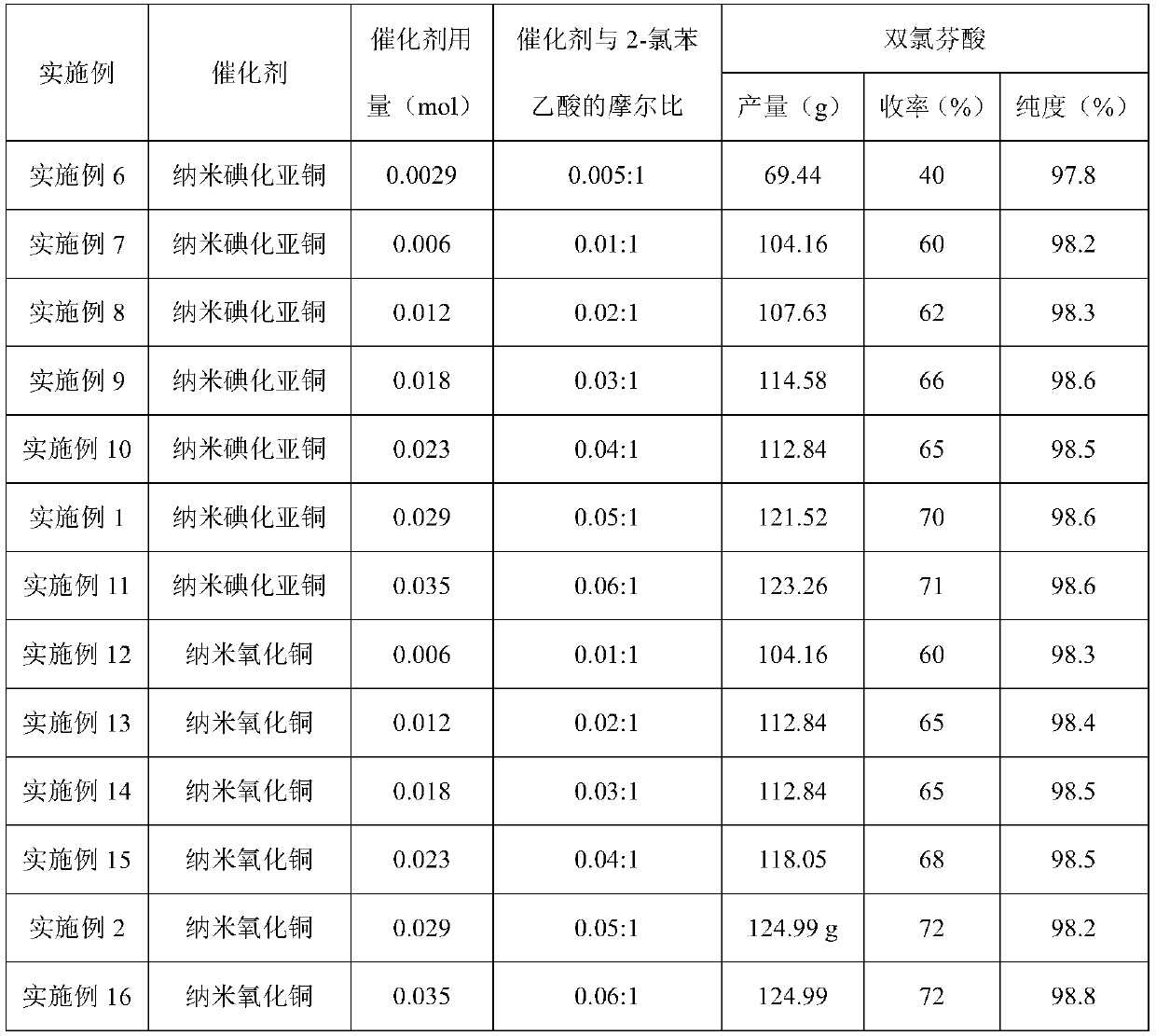
Embodiment 1
[0028] A kind of preparation method of diclofenac is characterized in that, comprises the following steps:
[0029] (1) Add 2-chlorophenylacetic acid (100g, 0.586mol) and N,N-dimethylformamide (600ml) into a 3-port dry glass bottle, stir and dissolve, then add 2,6-dichloroaniline ( 142.41g, 0.879mol), potassium carbonate (161.97g, 1.172mol), potassium iodide (92.28g, 0.586mol) and catalyst (0.029mol), heated to 130°C-150°C under the protection of nitrogen, kept at reflux and stirred for 7h; , the catalyst is nano-cuprous iodide particles; the nano-cuprous iodide is prepared according to the method recorded in the literature (The Journal of Organic Chemistry 2011, 76, 2296-2300).
[0030] (2) After cooling the reaction mixture obtained in step (1) to 80° C., heat filter it with diatomaceous earth, collect the filtrate, wash the filter cake with hot water at 80° C., collect the lotion, combine the filtrate and the lotion, and distill off the water and the lotion under reduced pr...
Embodiment 2
[0032] The content of Example 2 is basically the same as that of Example 1, except that the catalyst is nano-copper oxide particles, and the nano-copper oxide is purchased from Aldrich.
Embodiment 3
[0034] The content of embodiment 3 is basically the same as embodiment 1, and its difference is: catalyst is nanometer cuprous oxide coating copper particle; 779) prepared by the method described.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com