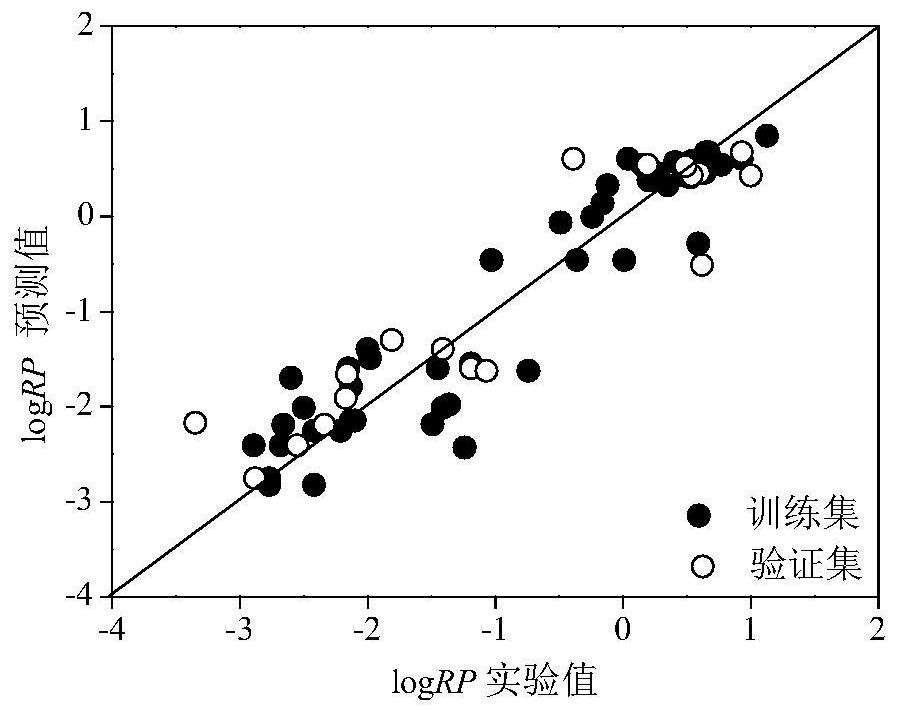
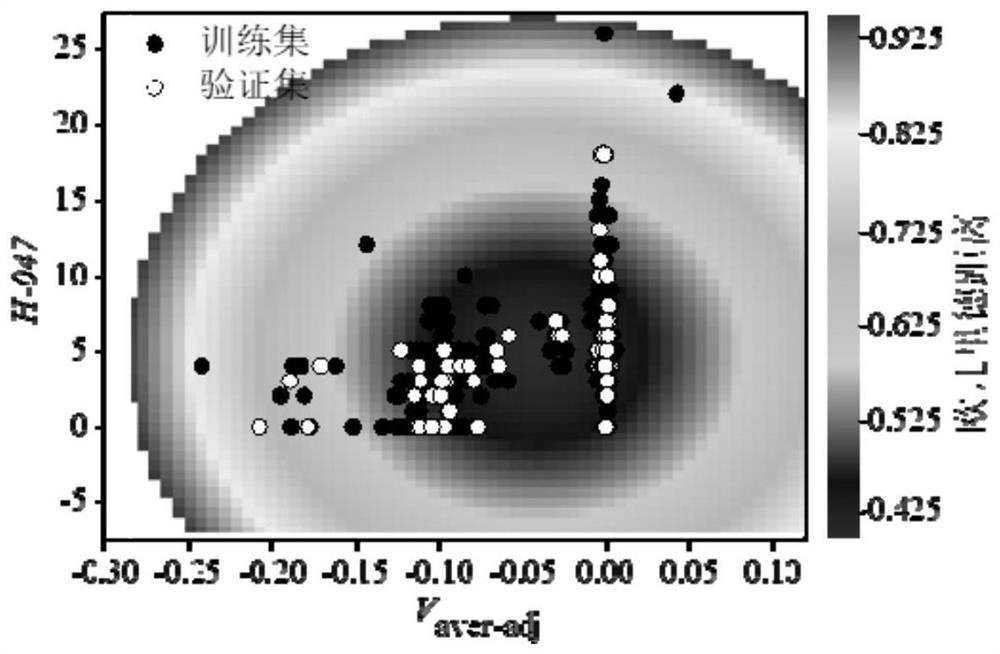
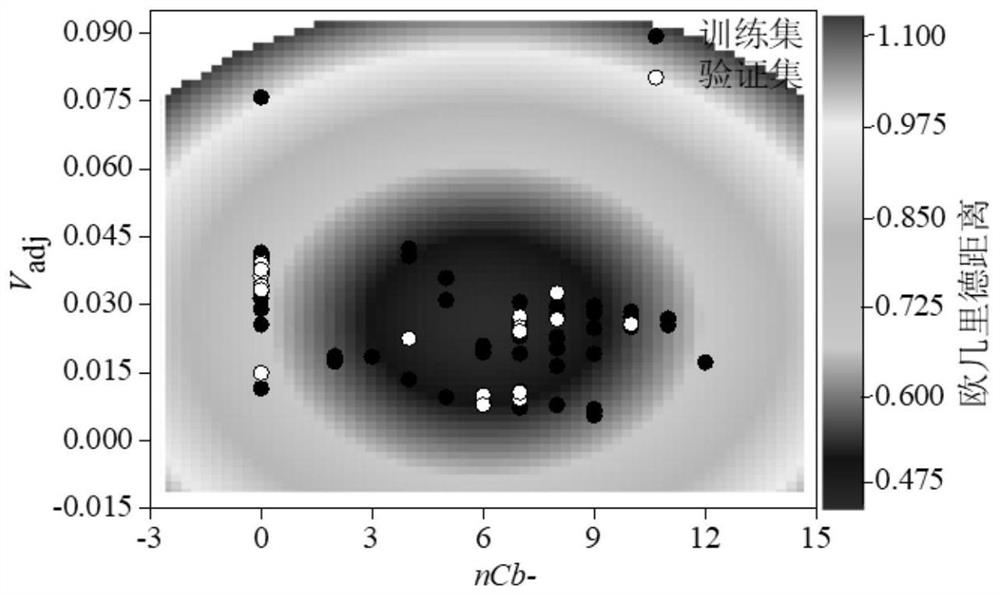
Method for Screening Human Transthyretin Interferors Using K-Nearest Neighbor Algorithm
A technology of thyroxine and transport protein, applied in computing, computer components, instruments, etc., can solve problems such as unpredictable interference effects, and achieve the effects of easy programming, clear mechanism, and good scalability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 2,3,3',5,5'-Pentachlorobiphenyl has no hTTR interference activity. The steps of utilizing the present invention to predict its interference activity are as follows:
[0071] Calculate the descriptor required for the classification model according to Gaussian 16 and Dragon 6.0, namely V aver-adj (morphologically corrected average molecular electrostatic potential), F-083 (fluorine atom attached to sp3 hybridized carbon atom), H-047 (hydrogen atom attached to sp3 hybridized or sp2 hybridized carbon atom). Then its Euclidean distance is calculated to be 0.191, which is within the application domain of the binary classification model (Euclidean distance is less than 0.928). Therefore, a binary classification model can be used to distinguish the interfering activity of 2,3,3',5,5'-pentachlorobiphenyl on hTTR. According to the descriptors of organic chemicals and 2,3,3',5,5'-pentachlorobiphenyl in the binary classification model training set, the kNN algorithm based on Eucl...
Embodiment 2
[0073] 4'-HO-3,3',4,5,5'-pentachlorobiphenyl has hTTR interference activity (logRP=0.933). The steps of utilizing the present invention to predict its interference activity are as follows:
[0074] According to Gaussian 16 and Dragon 6.0, calculate the required descriptor for the required classification model, namely V aver-adj (morphologically corrected average molecular electrostatic potential), F-083 (fluorine atom attached to sp3 hybridized carbon atom), H-047 (hydrogen atom attached to sp3 hybridized or sp2 hybridized carbon atom). Then its Euclidean distance is calculated to be 0.187, which is within the application domain of the binary classification model (Euclidean distance is less than 0.928). Therefore, a binary classification model can be used to distinguish the interfering activity of 4'-HO-3,3',4,5,5'-pentachlorobiphenyl on hTTR. According to the descriptors of organic chemicals and 4'-HO-3,3',4,5,5'-pentachlorobiphenyl in the binary classification model traini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com